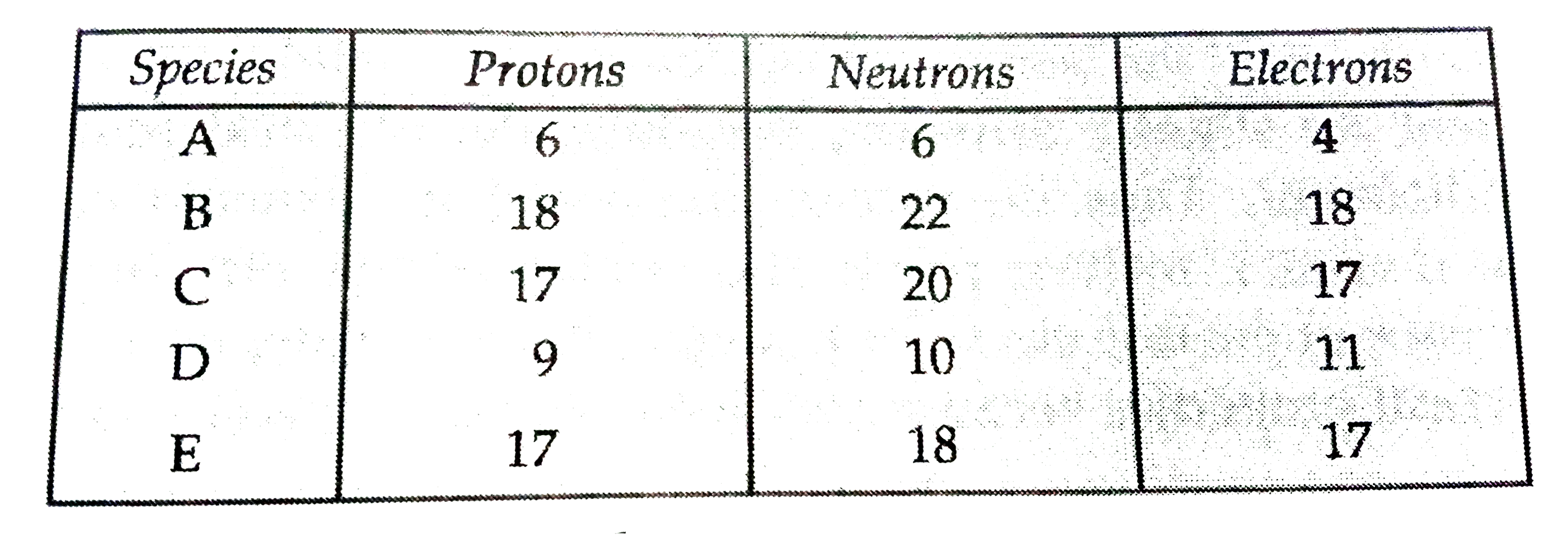
The number of protons neuitrons and electrons in particles from A to E are given below:
(i) Which one is a cation? , (ii) which one is an anion?
Which represent pair of isoptopes?
Video Solution
Text Solution
(i) NB is a monovalent cation (B+) , (ii) E is a monovalent anion (E−)
(iii) A and D represent pari of isotopes
|
Similar Questions
Knowledge Check
Which one of the following pairs represent stereoisomerism?
AStructural and geometrical isomerismBLinkage and geometrical isomerismCChain and rotational isomerismDOptical and geometrical isomerismWhich one of the following pairs represents isotopes?
A3517Cl,3717ClB21H,32HeC21H,32HeD7632Ge,7634SeWhich one of the following pair represents stereo isomerism :-
ALinkage isomerism and Geometrical isomerismBChain isomerism and Rotational isomerismCOptical isomerism and Geometrical isomerismDStructural isomerism and Geometrical isomerism
Give one example of (i) a polyatomic cation, (ii) a polyatomic anion.
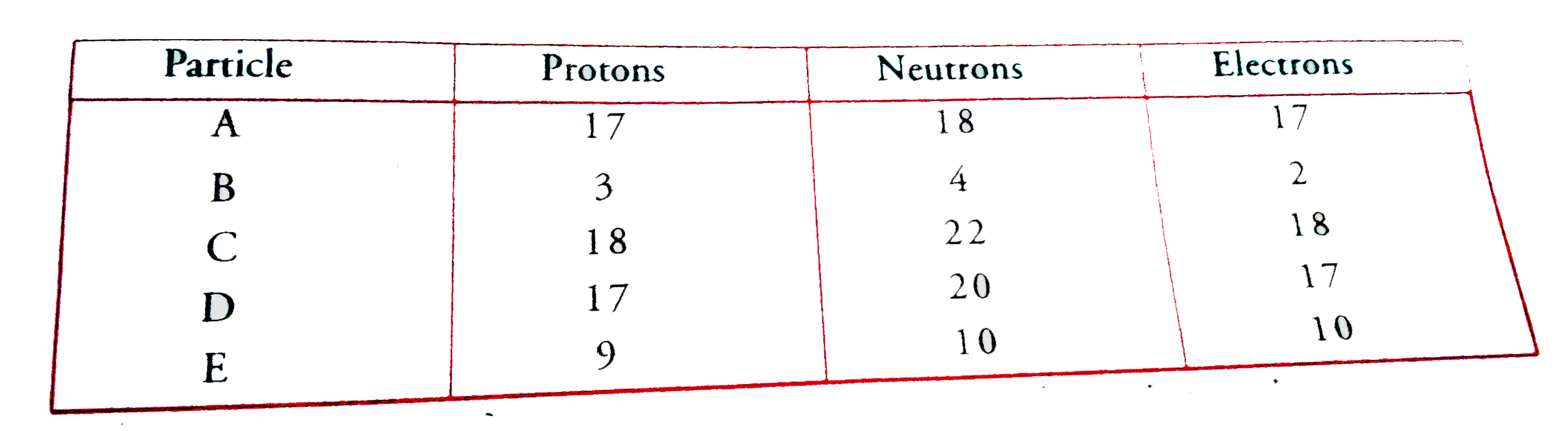
View SolutionThe number of protons, neutrons and electrons in particles A to E are given below :
ParticleProtonsNeutronsElectronsA171817B342C182218D172017E91010
Giving reasons, find a pair of isotopes from the above particles.
View SolutionWhich one of the following pairs represent stereoisomerism?
View SolutionWhich one of the following pair represents isobars?
View Solutionwhich one of the following pairs represents the stereoisomerism?
View Solution