The under certain conditions magnesium can be reduce SiO2 and silicon can reduce MgO. What are those conditions?
Video Solution
Text Solution
Below the melting point of silicon (1693 K), the ΔfG∘ curve for the formation of SiO2 lies above the ΔfG∘ curve for Mgo. Therefore, below 1693 K, Mg can reduce SiO2 to Si. However, above 1693 K, the ΔfG∘ curve for MgO lies above ΔfG∘ curve for SiO2 and therefore, at temperatures above 1693 K, Si can reduce MgO to Mg.
|
Similar Questions
Knowledge Check
A can reduce BO2 under which conditions.
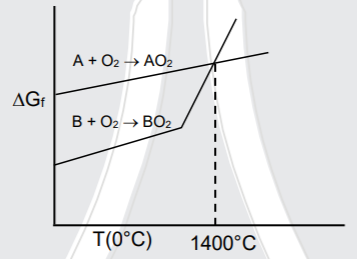 A>1400∘CB<1400∘CC>1200∘C and <1400∘CD<1200∘C
A>1400∘CB<1400∘CC>1200∘C and <1400∘CD<1200∘CSO2 can reduce :
AHCIO3 to HClBCr2O2−7/H+ to Cr3+CMnO−4/H+ to Mn2+DIO−3 to I2Air friction can be reduced by making the shape of the objects
Athe use of lubricants.Bpolishing surfaces.Cby increasing the height.DBoth (a) and (b)
Is it true that certain conditions Mg can reduce SiO2 and Si can reduce MgO? What are those conditions?
View SolutionThe under certain conditions magnesium can be reduce SiO2 and silicon can reduce MgO. What are those conditions?
View SolutionIs it true that under certain conditions, Mg can reduce SiO2, and Si can reduce MgO ?
View SolutionIt is true that under certain condition, Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
View SolutionSoil erosion can be reduced by:
View Solution
