Though coke is made from coal, it is used for metallurgical processes instead of coal. Give reason.
Step-by-Step Solution:
1. Understanding the Composition of Coke and Coal:
- Coke is derived from coal through a process called carbonization, where coal is heated in the absence of air. This process removes volatile substances and leaves behind a solid residue known as coke.
- Coal, on the other hand, contains a mixture of various substances, including carbon, hydrogen, sulfur, oxygen, and nitrogen.
2. Coke's Purity and Carbon Content:
- The resulting coke is primarily composed of carbon, with about 98% carbon content. This high purity makes it an excellent reducing agent in metallurgical processes.
- In contrast, coal has a lower carbon content (typically around 60-80%) and contains impurities such as coal tar and coal gas.
3. Importance in Metallurgical Processes:
- In metallurgical processes, especially in the production of iron and steel, a high carbon content is crucial. The carbon in coke reacts with iron ore to produce molten iron and carbon dioxide.
- The presence of impurities in coal can lead to unwanted reactions and lower the efficiency of the metallurgical process.
4. Conclusion:
- Therefore, coke is preferred over coal in metallurgical processes due to its higher carbon content and purity, which ensures better efficiency and quality in metal production.
- Coke is derived from coal through a process called carbonization, where coal is heated in the absence of air. This process removes volatile substances and leaves behind a solid residue known as coke.
- Coal, on the other hand, contains a mixture of various substances, including carbon, hydrogen, sulfur, oxygen, and nitrogen.
- The resulting coke is primarily composed of carbon, with about 98% carbon content. This high purity makes it an excellent reducing agent in metallurgical processes.
- In contrast, coal has a lower carbon content (typically around 60-80%) and contains impurities such as coal tar and coal gas.
- In metallurgical processes, especially in the production of iron and steel, a high carbon content is crucial. The carbon in coke reacts with iron ore to produce molten iron and carbon dioxide.
- The presence of impurities in coal can lead to unwanted reactions and lower the efficiency of the metallurgical process.
- Therefore, coke is preferred over coal in metallurgical processes due to its higher carbon content and purity, which ensures better efficiency and quality in metal production.
Similar Questions
Knowledge Check
Coke is used in metallurgical process chiefly as
AFluxBreducing agentCslagDoxidising agentCoal tar is used in manufacture of:
Asynthetic dyesBdrugsCexplosivesDall of these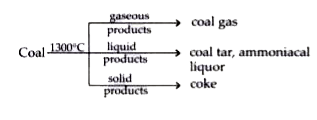
The most important use of coal tar isAit is a source of aromatic hydrocarbonsBit is a good reducing agent for extraction of ironCit is used as a fuelDit is used for making shoe polish.