Three elements P, Q, and R have successive atomic numbers with same period . If P > R > Q in terms atomic size. Predict the group of each element. Give reason. Solution in Kannada
Since P, Q, and R have successive atomic numbers, they lie in the same period. We know that even though atomic size decreases along a period, inert-gas elements have a slightly bigger atomic size than that of their preceding element. Hence, R must be an inert gas. Hence, the group numbers of P. Q. and Rare 16, 17, and 18 respectively.
Similar Questions
Atomic number of three elements P, Q and R are 20, 38 and 56 elements respectively. Which option is correct?
View Solutionतीन तत्व P, Q तथा R घनीय ठोस जलक के रूप में क्रिस्टलीकृत होती है। P परमाणु कोनों पर, Q परमाणु घन के केन्द्र पर तथा R परमाणु किनारों पर उपस्थित है। योगिक का सूत्र क्या है -
View SolutionIf three elements P, Q and R crystallise in a cubic unit cell with P atoms at the corners, Q atoms at the cubic centre and R atoms at the centre of each face of the cube, then write the formula of the compound.
View SolutionThe atomic number of elements P, Q, R, S and T are given. Which element among these is a non metal?
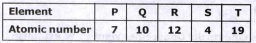
View SolutionThe atomic number of elements P, Q, R, S and T are given. Which element belongs to 3rd period of periodic table?
View SolutionThe atomic number of elements P, Q, R, S and T are given. Which two elements are chemically similar?
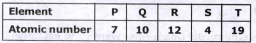
View SolutionThree elements P,Q and R crystallize in a cubic solid lattice. The P atoms occupy the corners. Q atoms the cube centres and R atoms the edges. The formula of the compound is
View Solution