Trans isomer is more stable than cis isomer. Justify this statement.Solution in Tamil
Trans isomer is more stable than cis isomer. This is because in the cis isomer, the bulky groups are on the same side of the double bond. The steric repulsion of the groups makes the cis isomers less stable than the trans isomers in which bulky groups are on the opposite side.
Similar Questions
How many statements are true for the following pair of compounds ?
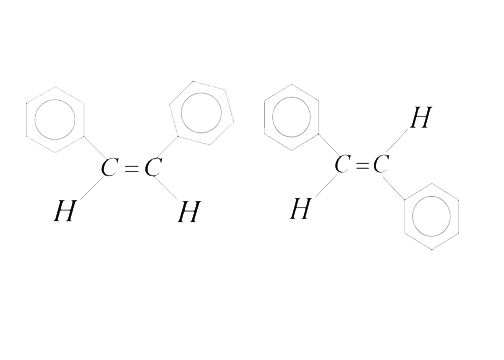
Cis Trans
(i) The dipole moment of trans isomer is zero
(ii) The boiling point of cis isomer is more than trans isomer
(iii) Cis isomer is more stable than the trans isomer
(iv) These are also called configurational diastereomers
(v) These are readily interconvertible under normal conditions
(vi) The melting point of trans isomer is more than the cis isomer
(vii) Trans isomer is more soluble than cis isomer in polar solvents
View SolutionC is isomers are less stable than trans isomers
View SolutionThe dipole moment of cis C_(2)H_(2)Cl_(2) isomer is more than that of trans isomer.
View SolutionCis isomer has lesser stability than the corresponding trans isomer
View SolutionStatement (S) : Polarity of cis isomer is more with respect to trans isomers.
Reason (R) : Cis and trans isomers are geometrical isomers.
View SolutionExplain why cis-isomer is less stable as compared to trans isomer
View SolutionOut of cis and trans isomers, which isomer is more stable ?
View Solution

