Use the data given in the following table to calculate the molar mass of naturally occuring argon isotopes :

¯¯¯A=Σfi×Ai
=(0.00337×35.96755)+(0.00063×37.9672)+(0.99600×39.9624)
=0.121+0.024+39.803
=39.948 g/mol
Similar Questions
Use data given in the following table to calculate the molar mass of naturaly occuring argon isotopes:
IsotopeIsotopic molar massAbundance.36Ar35.96755gmol−10.337%.38Ar37.96272gmol−10.063%.40Ar39.9624gmol−199.600%
View SolutionUse data given in the following table to calculate the molar mass of naturaly occuring argon isotopes:
IsotopeIsotopic molar massAbundance.36Ar35.96755gmol−10.337%.38Ar37.96272gmol−10.063%.40Ar39.9624gmol−199.600%
(Round of the answer to the nearest whole number ).
View Solution
Knowledge Check
Use the data given in the following table to calculate the molar mass of naturally occurring agfon.
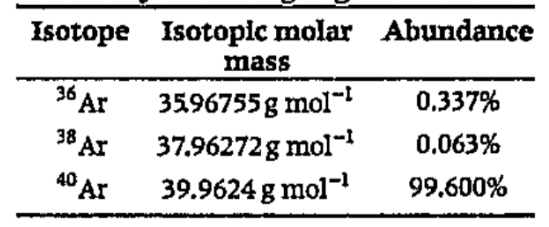
The molar mass of argon isA39.948B39.665C38.678D38.442
Use data given in the following table to calculate the molar mass of naturaly occuring argon isotopes:
IsotopeIsotopic molar massAbundance.36Ar35.96755gmol−10.337%.38Ar37.96272gmol−10.063%.40Ar39.9624gmol−199.600%
(Round of the answer to the nearest whole number ).
View SolutionUse data given in the following table to calculate the molar mass of naturaly occuring argo isotopes:
IsotopeIsotopic molar massAbundance.36Ar35.96755gmol−10.337%.38Ar37.96272gmol−10.063%.40Ar39.9624gmol−199.600%
View SolutionCalculate the molar mass of ammonia.
View SolutionUsing the following data, calculate COE :

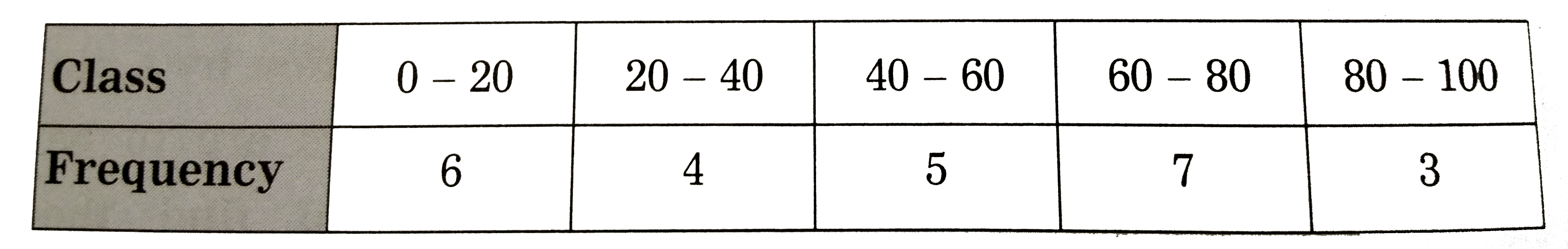
View SolutionFind the mean of the data given in the following table :
View SolutionUsing the following data calculate the compensation of employees :

View SolutionDraw a bar graph using the data given in the following table.

View Solution

