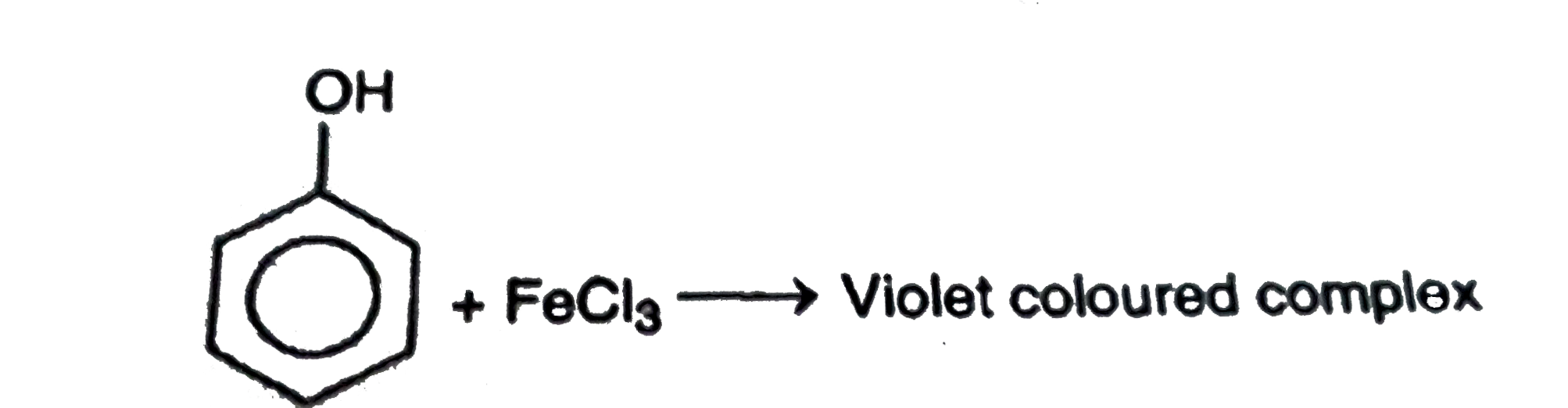 Violet coloured complex
Violet coloured complex
The formula of the complex formed in the above reaction, is
A
[Fe(OC6H5)3]3−
B
[Fe(OC6H5)3]3+
C
[Fe(OC6H5)6]3−
D
[Fe(OC6H5)6]3+
Text Solution
The correct Answer is:C
Fe(OC6H5)6]3−
|
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBook:BITSAT GUIDEChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:BITSAT Archives
Explore 14 Videos
- ADSORPTION AND COLLOIDAL SYSTEMBook:BITSAT GUIDEChapter:ADSORPTION AND COLLOIDAL SYSTEMExercise:BITSAT Archives
Explore 5 Videos
- ALDEHYDE AND KETONESBook:BITSAT GUIDEChapter:ALDEHYDE AND KETONESExercise:Bitsat Archives
Explore 14 Videos
Similar Questions
Knowledge Check
Correct formula of the complex formed in the brown ring test for nitrates is
AFeSO4NOB[Fe(H2O)5NO]2+C[Fe(H2O)5NO]+D[Fe(H2O)5NO]3+Correct formula of the complex formed in the brown ring test for nitrates is
AFeSO4.NOB[Fe(H2O)5NO]2+C[Fe(H2O)5NO]+D[Fe(H2O)5NO]3+Correct formula of the complex formed in the brown ring test for nitrates is
AFeSO4.NOB[Fe(H2O)5NO]2+C[Fe(H2O)5NO]+D[Fe(H2O)NO]3+
The formula of the complex ethylenetrichloridoplatinum (II) is
View SolutionThe colour of a complex compound is due to .
View SolutionFor a complex reaction :
View Solutionviolet coloured complex obtained in the detectiomn of sulphur is :
View SolutionViolet coloured complex obtained in the detection of sulphur is:
View Solution

