What are the examples of separation of mixture on the basis of their densities?
Step-by-Step Solution
1. Understanding Density: Density is a property of matter that describes how much mass is contained in a given volume. In mixtures, components can have different densities, leading to the formation of layers when allowed to settle.
2. Formation of Layers: When two liquids of different densities are mixed, the denser liquid will settle at the bottom, forming the lower layer, while the less dense liquid will float on top, forming the upper layer.
3. Example 1 - Carbon Tetrachloride and Water:
- When carbon tetrachloride (CCl₄) is mixed with water, carbon tetrachloride, which has a higher density than water, will form the lower layer.
- Water, being less dense, will form the upper layer.
- This separation can be observed visually, and the two layers can be separated using a separating funnel or similar apparatus.
4. Example 2 - Extraction of Iron from Blast Furnace:
- In the process of extracting iron, a mixture of molten iron and slag is produced.
- The molten slag, which has a lower density, will float on top of the molten iron, which has a higher density.
- These two layers can be separated through holes in the furnace, allowing the slag to be removed from the top and the molten iron to be collected from the bottom.
5. Conclusion: The separation of mixtures based on density can be effectively demonstrated through the examples of carbon tetrachloride and water, as well as the extraction of iron from a blast furnace.
- When carbon tetrachloride (CCl₄) is mixed with water, carbon tetrachloride, which has a higher density than water, will form the lower layer.
- Water, being less dense, will form the upper layer.
- This separation can be observed visually, and the two layers can be separated using a separating funnel or similar apparatus.
- In the process of extracting iron, a mixture of molten iron and slag is produced.
- The molten slag, which has a lower density, will float on top of the molten iron, which has a higher density.
- These two layers can be separated through holes in the furnace, allowing the slag to be removed from the top and the molten iron to be collected from the bottom.
टॉपर्स ने हल किए ये सवाल
- IS MATTER AROUND US PURE ?BOOK - MTG IIT JEE FOUNDATIONCHAPTER - IS MATTER AROUND US PURE ?EXERCISE - SOLVED EXAMPLES
34 Videos
- IS MATTER AROUND US PURE ?BOOK - MTG IIT JEE FOUNDATIONCHAPTER - IS MATTER AROUND US PURE ?EXERCISE - NCERT SECTION
45 Videos
- FOOTSTEPS TOWARDS(CBSE BOARD)BOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS(CBSE BOARD)EXERCISE - SECTION-D
14 Videos
- MATTER IN OUR SURROUNDINGS BOOK - MTG IIT JEE FOUNDATIONCHAPTER - MATTER IN OUR SURROUNDINGS EXERCISE - OLYMPIAD/HOTS CORNER
9 Videos
समान प्रश्न
How will you separate a mixture of common salt, sulphur powder and sand?
View SolutionWhat is meant by a pure substance?
View SolutionWhy did Rutherford select a gold foil in his α-ray scattering experiment ?
View SolutionDry ice is an example of ______ process.
View SolutionMention the purposes for which separation of mixtures is important Give examples.
View Solutionमोनेरा को किस आधार पर अलग किया गया है ?
View Solutionअक्रिय गैसों के मिश्रण का पृथक्करण किस प्रकार के चारकोल द्वारा किया जाता है ? इसका आधार क्या है ?
View Solutionअक्रिय गैसों के मिश्रण का पृथक्करण किस प्रकार के चारकोल द्वारा किया जाता है ? इसका आधार क्या होता है ?
View SolutionDiscuss on what basis we will operate on which method is suitable for separating a mixture
View SolutionWhat is the basis for choosing a separation technique to separate mixtures ?
View SolutionWhat is the basis for choosing a separation technique to separate mixtures
View Solution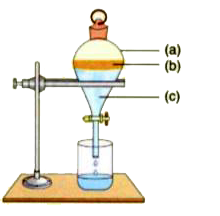
Give one example of a mixture which can be separated using this method.
View SolutionWhat is the basis of separation in chromatography ?
View SolutionDescribe an example for the separation of components of mixture by sublimation.
View SolutionDescribe an example for the separation of a mixture by the process of evaporation.
View SolutionOn what priciple are the following methods of separation based? Give on example of a mixture of the methods mentioned in which they are used.
Magnetic separations.
View Solution
