What changes occur when acidified Cr2O2−7 ion react with H2O2 solution in presence of ether solvent?
Orange colour of solution turns blue
Oxidation state of Cr-atom decrease
Oxidation state of Cr-atom remains constant
Orange colour of solution turns green
The correct Answer is:A, C
Step by step video solution for What changes occur when acidified Cr_(2)O_(7)^(2-) ion react with H_(2)O_(2) solution in presence of ether solvent? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- d-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - d-BLOCK ELEMENTSEXERCISE - MATCH THE COLUMN
3 Videos
- d-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - d-BLOCK ELEMENTSEXERCISE - ASSERTION-REASON TYPE QUESTIONS
31 Videos
- d-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - d-BLOCK ELEMENTSEXERCISE - Level 3
36 Videos
- CO-ORDINATION COMPOUNDSBOOK - VK JAISWAL ENGLISHCHAPTER - CO-ORDINATION COMPOUNDSEXERCISE - SUBJECTIVE PROBLEMS
42 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - VK JAISWAL ENGLISHCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - ASSERTION-REASON TYPE QUESTIONS
14 Videos
Similar Questions
Entries of Column-I are to be matched with entries of Coloumn-II. Each entry of Column-I may have the matching with one or more than one entries of Column-II.
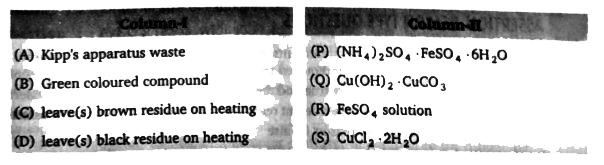
View SolutionAssertion: Melting point of Mn is more than that of Fe.
Reason : Mn has higher number of unpaired e2 than Fe in atomic state.
View SolutionIn salts of polyatomic anion, as polarising power of cation increase, thermal stability of the salt decrease and decomposed species may further undergo redox reaction
Q. Which of the followinng species undergoes non-redox thermal decomposition reaction on heating?
View SolutionConsider the following compounds with their regular geometries.

Calculate value of (x+y−z)2, where x is total number of axial d-orbitals having zero nodal plane, y and z are total no. of non-axial and axial d-orbitals respectively each one having two nodal planes used in hybridisation of central atoms of compounds A to E.
View SolutionAssertion (A) Green house effect was observed in houses used to grow plants and these are made of green glass.
Reason (R) Green house name has been given because glass houses are made of green glass.
View SolutionWhen H_(2)S is passed through acidified K_(2)Cr_(2)O_(7) solution, the solution turns :
View SolutionAcidified solution of K_(2)Cr_(2)O_(7) on treatment with H_(2)O_(2) yields :
View SolutionWhat happens when H_(2)O_(2) is shaken with acidified K_(2)Cr_(2)O_(7) solution ?
View SolutionWhat happens when H2O is treated with acidified solution of K2Cr2O7 containing some ether?
View SolutionWhen H_(2)O_(2) is added to a acidified solution of K_(2)Cr_(2)O_(7) :
View SolutionAcidified K_(2)Cr_(2)O_(7) reacts with H_(2)O_(7) to give "…............." coloure due to the formation of "…..................." .
View SolutionWhen H2O2 is shaken with an acidified solution of K2Cr2O7 in the presence of ether , the ethereal layer turns blue due to the formation of
View SolutionWhat changes occur when acidified CrO2−4 ion react with H2O2 solution in presence of ether solvent?
View SolutionWhen H_(2)O_(2) is shaken with an acidified solution of K_(2)Cr_(2)O_(7) in presence of ether, the ethereal layer turns blue due to the formation of
View SolutionWhen H2O2 is shaken with an acidified solution of K2Cr2O7 is presence of ether, layer turns blue due to the formation of-
View SolutionWhen H2O2 is shaken with an acidified solution of K2Cr2O7 is presence of ether, layer turns blue due to the formation of-
View Solution

