What Is a periodic property ? How do the following properties change in a group and period ? Explain .
Ionization enery Solution in Telugu
Periodic property :Periodicity means that when elements are arranged accoding to their electronic configuratiin , the properties of elements in the perioodic table reoccr at regular intervals - such properties which reoccur are called called as periodic properties
change of properties in a group and a period :
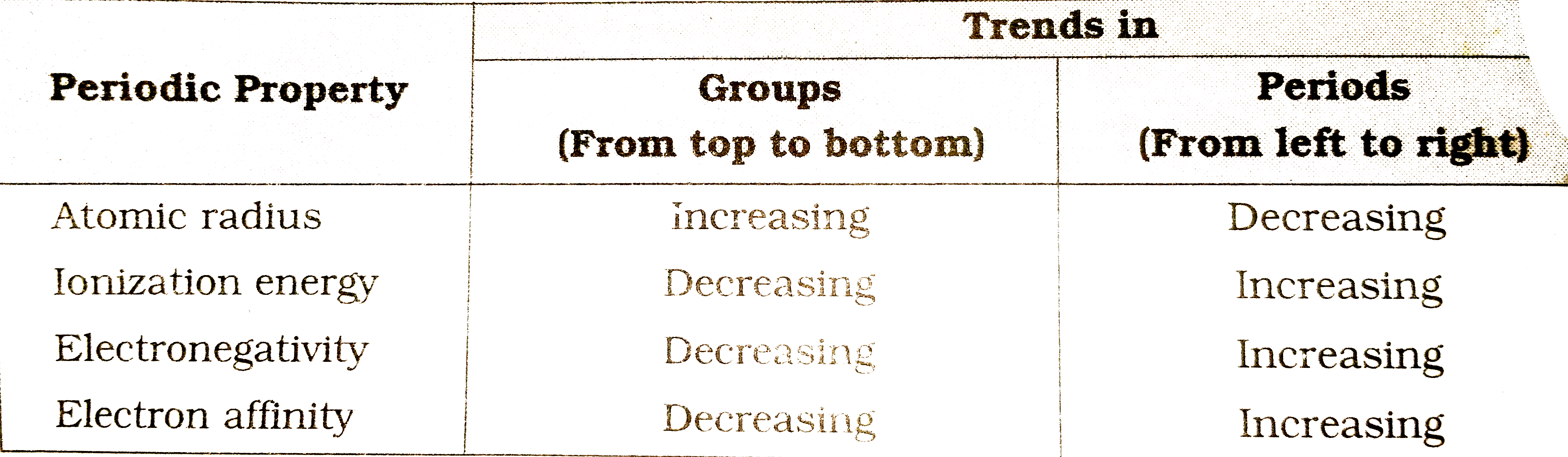
Ionization energy : The energy required to remove an electron from the outermost orbit or shell of a neutral gaseous atom is called ionization energy .
Variation in group : As we go down in a group atomic radius increases . hence the ionization energy decreases from top to bottom .
Variation in period : in period the value of ionization enthalpy increases from left to right with breaks where the atoms have somewhat stable configurations .
Similar Questions
How do the following properties change in a group and in periods? Atomic radius
View SolutionHow do the following properties change in a group and in periods? Ionization enthalpy
View SolutionHow do the following properties change in a group and in periods? Debt electrification
View Solutionక్రింది ధర్మాలు గ్రూపులో మరియు పీరియడ్ లలో ఏ విధంగా మారుతాయి? ఎలక్ట్రాన్ గ్రాహ్య ఏంథాల్పీ
View SolutionWhat is a periodic property? How the following properties vary in a group and in a period? Expain
(a) Atomic radius.
View SolutionWhat is a periodic property? How the following properties vary in a group and in a period? Expain
(b) Electron gain enthalpy.
View SolutionWhat is a periodic property? How the following properties vary in a group and in a period? Explain
(a) IP.
View SolutionWhat is a periodic property? How the following properties vary in a group and in a period? Explain
(b) EN.
View Solution
