What is maximum electrons in that can be accommodated in L shell.Solution in Malayalam
The correct Answer is:8
Step by step video & text solution for What is maximum electrons in that can be accommodated in L shell. by Chemistry experts to help you in doubts & scoring excellent marks in Class 9 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
What is the relation between the total mass of the reactants and the total mass of products ?
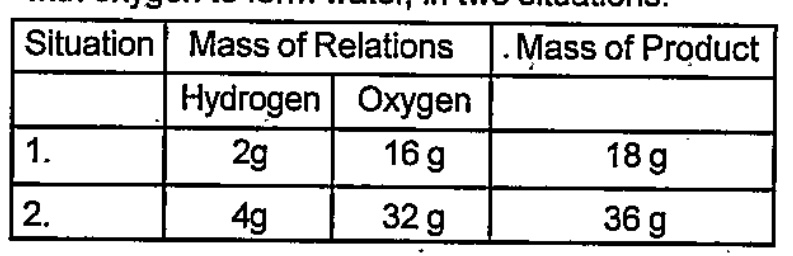
View SolutionWhat is the maximum number of electrons an M shell of an atom can accommodate ?
View SolutionWhat is the maximum number of electrons that can be accommodated in a shell? Write formula.
View SolutionMaximum number of electrons that can be accommodated in N shell is
View SolutionMaximum of number of electrons that can be accommodated in M - shell is
View SolutionThe maximum number of electrons that can be accommodated in the L-shell of an atom is
View SolutionWhat is the maximum number of electrons that can be accommodated in a shell? Write the formula.
View Solutionwhat is the maximum number of electrons that can be accommodated in a shell ?
View SolutionThe maximum number of electrons that can be accommodated in M Shell is
View SolutionWhat is the maximum number of electrons which can be accommodated in ‘N’ shell?
View SolutionWhat is the maximum number of electrons which can be accommodated in ‘N’ shell?
View SolutionThe maximum number of electrons that can be accommodated in N shell is………………..
View SolutionThe maximum number of electrons that can be accommodated in F shell is………………..
View SolutionWhat is the maximum number of electrons that can be accommodated in L shell? (L shell or 2nd orbit)
View SolutionWhat is maximum electrons that can be accommodated in K shell.
View Solution
