What is the type of hybridisation of each carbon in the following compounds?
(a) CH3Cl (b) (CH3)2CO (c ) CH3CN (d) HCONH2 (e ) CH3CH=CHCN
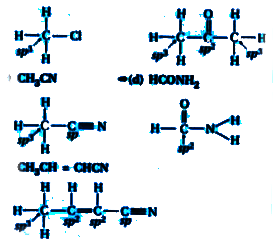
sp3 carbon has four single bond
sp2 carbon has one double bond
sp carbon has one triple bond or two double bond
Topper's Solved these Questions
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBOOK - KUMAR PRAKASHANCHAPTER - ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESEXERCISE - Section A -Try Your Self-3
20 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBOOK - KUMAR PRAKASHANCHAPTER - ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESEXERCISE - Section-B (Short Questions)
282 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBOOK - KUMAR PRAKASHANCHAPTER - ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESEXERCISE - Section -A(Quantitative Analysis)
18 Videos
- HYDROGENBOOK - KUMAR PRAKASHANCHAPTER - HYDROGENEXERCISE - Section-D NCERT Exemplar (Long Answer)
8 Videos
- QUESTION ASKED IN JEE -2018BOOK - KUMAR PRAKASHANCHAPTER - QUESTION ASKED IN JEE -2018EXERCISE - MCQ
10 Videos
Similar Questions
38cm2N2 gas at 300K temperature and 96pa pressure. In 0.25g organic compound calculate the % of nitrogen. (1 atm .- 101.3 pa)
View SolutionWhat is catenation?
View SolutionExplain the principle and estimation method for carbon and hydrogen in organic compound
View SolutionAtomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
View SolutionAccording to molecular orbital theory, which of the following will not be a viable molecule?
View SolutionWhat is the type of hybridzation of each carbon in the following compounds? CH_(3)Cl (b) (CH_(3))_(2)CO (c ) CH_(3)CN (d) HCONH_(2) (e) CH_(3)CH=CHCN
View SolutionState the nature of hybridisation of each carbon atom in the following compounds ?
(a)CH3Cl (b)(CH3)2C=O (c)CH3−C≡N (d)H−O||C−NH2 (e)CH3−CH=CH−C≡N
View SolutionWhat is the type of hybridzation of each carbon in the following compounds?
CH3Cl (b) (CH3)2CO (c ) CH3CN (d) HCONH2 (e) CH3CH=CHCN
View SolutionWhat are hybridisation states of each carbon atom
in the following compounds ?
CH2=C=O,CH3CH=CH2,(CH3)2CO,<br>CH2=CHCN,C6H6.
View SolutionWhat is the type of hybridisation of each carbon in the following compounds? (a) CH_(3)-CH_(3) (ii) (CH_(3))_(2)CO
View Solutionनिम्नलिखित यौगिकों में प्रत्येक कार्बन की संकरण अवस्था बताइए-
CH2=C=O,CH3CH=CH2,(CH3)2CO,CH2=CHCN,C6H6
View SolutionWhat is the type of hybridisation of each carbon in CH_(3) - CH = CHCN
View SolutionWhat are hybridisation states of each carbon atom in the following compounds ? CH2=C=O,CH3CH=CH2, (CH3)2CO, CH2=CHCN and HCONH2
View SolutionWhat are hybridisation states of each carbon atom in the following compounds?
CH2=C=O,CH3CH=CH2,(CH3)2CO,CH2=CHCN,C6H6
View SolutionWhat is the type of hybridisation of each carbon in the following compounds?
(a) CH3Cl, (b) (CH3)2CO, (c ) CH3CN,
(d) HCONH2, (e) CH3CH=CHCN
View SolutionWhat are hybridisation states of each carbon atom in the following compounds ?
CH2=C=O,CH3CH=CH2,(CH3)2CO,CH2=CHCN,C6H6
View Solution

