Which is the correct name of the following structure? Why? CH3−CH2−CH∣CH2−CH3−CH2−CH2−CH∣CH3−CH2−CH3
(i) 3-ethyl-6-methyloctane (ii) 3-methyl-6-ethyloctane (iii) 6-methyl-3-ethyloctane
(i) Name is correct because, form both the order of carbon is same, so, methyl group has 6 no. and ethyl group has 3 no. as per alphabetical order.
Name (iii) is wrong- because ethyl must be first than methyl
Topper's Solved these Questions
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section-B - Match the following
Explore 28 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section-B - State True or False For The Following Statements
Explore 42 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section A -Try Your Self-3
Explore 20 Videos
- HYDROGENBook:KUMAR PRAKASHANChapter:HYDROGENExercise:Section-D NCERT Exemplar (Long Answer)
Explore 8 Videos
- QUESTION ASKED IN JEE -2018Book:KUMAR PRAKASHANChapter:QUESTION ASKED IN JEE -2018Exercise:MCQ
Explore 10 Videos
Similar Questions
Match column-I and column-II with correct relation.

View Solution(i) Organic compound is first synthesised by F. Wohier
(ii) Berzeiius stated that some mysterious force existing in the living organism.
(iii) In 1882 Wohler prepared organic compound from inorganic compound at that time vital force they accepted.
(iv) Kolbe prepared methane and Berthelot prepared acetic acid
View Solution38cm2N2 gas at 300K temperature and 96pa pressure. In 0.25g organic compound calculate the % of nitrogen. (1 atm .- 101.3 pa)
View SolutionAtomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
View SolutionAccording to molecular orbital theory, which of the following will not be a viable molecule?
View SolutionWrite IUPAC name of the following compound (a) CH_(3)-CH_(2)-underset(CH_(3))underset(|)(CH)-CH_(2)underset(CH_(2)-CH_(3))underset(|)(CH)-CH_(2)-CH_(2)-CH_(3) (b) CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-CH_(2)-overset(CH_(2)-CH_(3))overset(|)(CH)-CH_(2)-underset(CH_(3))underset(|)(CH)-CH_(3) (c)
View SolutionWrite the IUPAc name of the following compound :
(a) CH3−CH2−CH3∣CH−CH=CH−CH2−CH3
(b) CH3−CH2−CH3∣CH−CH2−CH3∣CH−CH∣CH2∣CH2∣CH−CH3∣CH3−CH=CH3∣C−CH3
View SolutionStructures and IUPAC names of some hydrocarbons are given below. Explain why the names given in the parentheses are incorrect. underset(["and not 3,4,7-Trimethyloctane"])underset(2,5,6- "Trimethyloctane")(CH_(3)-underset(CH_(3))underset(|)(CH)-CH_(2)-CH_(2)-underset(CH_(3))underset(|)(CH)-underset(CH_(3))underset(|)(CH)-CH_(2)-CH_(3)) underset(["and not 5-Ethyl-3-methylheptane"])underset("3-Ethyl-5-methylheptane")(CH_(3)-CH_(2)-underset(CH_(2)CH_(3))underset(|)(CH)-CH_(2)-underset(CH_(3))underset(|)(CH)-CH_(2)-CH_(3))
View SolutionGive the IUPAC names of the following compounds:
(i)C6H5−CH2−CH2−OH (ii)CH3−CH3∣C∣CH3−CH2−CH2−OH
(iii)[(CH3)2CH]3COH. (iv)CH3−CH∣OH−CH2−CH2−O−CH2CH3
(v)CH3−CH2−CH∣Cl−CH∣CH3−CH3∣C∣OH−CH3 (vi)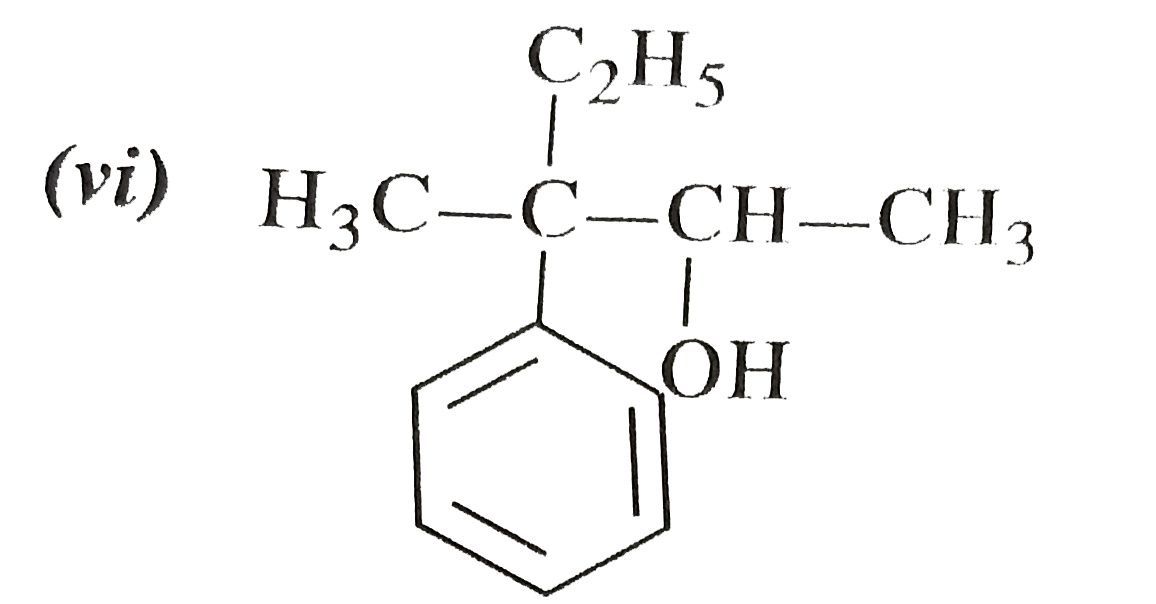
(vii)(CH3)3COH (viii)CH3−CH∣CH3−CH∣OH−CH2−CH∣CH3−CH3
(ix) CH2OH |CH2−CH2−CH−CH−CH−CH3 | | CH2Cl CH3, (x) CH3−C=C−CH2OH | | CH3Br
(xi) CH2=CH−CH∣OH−CH2−CH3, (xii) CH2OH |CH3−CH−CH2−CH−CH−CH3 | | CH3 OH
View SolutionThe IUPAC name of CH_(3)-underset(CH_(3))underset(|)CH-underset(CH_(3))underset(|)CH-CH_(2)CH_(2)-underset(CH_(3))underset(|)CH-CH_(3)
View SolutionGive the IUPAC name for the following alkane.
(a) CH3−CH∣CH2∣CH2−CH2−CH2−CH∣CH2∣CH2−CH3
(b) CH3−CH∣CH3−CH∣CH3−CH∣CH2∣CH3−CH2−CH2−CH2−CH3
View SolutionGive IUPAC names of the following compounds: (i) CH_(3) -underset (Cl) underset (|) (CH) - underset (CH_(3)) underset (|) (CH) - underset (CH_(3)) underset (|)(CH) - CH_(2)OH (ii) CH_(3) - underset (CH_(3)) underset (|) (CH) - O - CH_(2)CH_(3) (ii)
View SolutionName the following compounds according to IUPAC system. (i) CH_(3) - CH_(2) - underset (CH_(2)Cl) underset(|)(CH) - overset (CH_(2)OH) overset(|) (CH) - underset (CH_(3)) underset (|) (CH) - CH_(3) CH_(3)- underset (CH_(3)) underset(|) (CH) -CH_(2) - underset(OH) underset(|) (CH) - overset (CH_(2)OH) overset(|) (CH) - CH_(2) (iii) (iv) H_(2)C = CH - underset (OH)underset (|) (CH) - CH_(2) - CH_(2) - CH_(3) (v) CH_(3) - underset (CH_(3)) underset ( |) C = underset (Br)underset(|) C - CH_(2)OH
View SolutionThe IUPAC name of CH3−CH3∣C∣CH3−CH2−CH2−CH∣CH2∣CH3−CH3 is ............. .
View SolutionWrite the IUPAC names of the following compounds. (i) CH_(3)-underset(CH_(3))underset(|)(CH)-underset(CH_(2)-CH_(3))underset(|)(CH)-CH_(2)-CH_(3) (ii) CH_(3)-underset(CHO)underset(|)(CH)-CH_(2)-CH_(3)
View Solution

