Which of the following ions can be separated by using NH4Cl and NH4OH ?
Fe3+andCr3+
Cr3+andCo2+
Cr3+andAl3+
Al3+andBa2+
The correct Answer is:B, D
To determine which ions can be separated using ammonium chloride (NH₄Cl) and ammonium hydroxide (NH₄OH), we need to analyze the behavior of various cations when treated with these reagents.
1. Identify the Cations: The given pairs of cations are:
- (i) Fe³⁺ and Cr³⁺
- (ii) Cr³⁺ and Co²⁺
- (iii) Cr³⁺ and Al³⁺
- (iv) Al³⁺ and Ba²⁺
2. Understand the Group Reagents: Ammonium chloride and ammonium hydroxide are used to precipitate certain cations. They are specifically used for the third group of cations in qualitative analysis, which includes Fe³⁺, Cr³⁺, and Al³⁺.
3. Analyze Each Pair:
- Pair (i) Fe³⁺ and Cr³⁺: Both Fe³⁺ and Cr³⁺ are third group cations. When treated with NH₄Cl and NH₄OH, both will precipitate as their hydroxides (Fe(OH)₃ and Cr(OH)₃). Therefore, they cannot be separated.
- Pair (ii) Cr³⁺ and Co²⁺: Cr³⁺ is a third group cation, while Co²⁺ is a fourth group cation. When treated with NH₄Cl and NH₄OH, only Cr³⁺ will precipitate as Cr(OH)₃, while Co²⁺ remains in solution. Thus, they can be separated.
- Pair (iii) Cr³⁺ and Al³⁺: Both Cr³⁺ and Al³⁺ are third group cations. When treated with NH₄Cl and NH₄OH, both will precipitate (Cr(OH)₃ and Al(OH)₃). Therefore, they cannot be separated.
- Pair (iv) Al³⁺ and Ba²⁺: Al³⁺ is a third group cation, while Ba²⁺ is a second group cation. When treated with NH₄Cl and NH₄OH, only Al³⁺ will precipitate as Al(OH)₃, while Ba²⁺ remains in solution. Thus, they can be separated.
4. Conclusion: The ions that can be separated using ammonium chloride and ammonium hydroxide are:
- Cr³⁺ and Co²⁺
- Al³⁺ and Ba²⁺
Final Answer:
The ions that can be separated using NH₄Cl and NH₄OH are:
- (ii) Cr³⁺ and Co²⁺
- (iv) Al³⁺ and Ba²⁺
- (i) Fe³⁺ and Cr³⁺
- (ii) Cr³⁺ and Co²⁺
- (iii) Cr³⁺ and Al³⁺
- (iv) Al³⁺ and Ba²⁺
- Pair (i) Fe³⁺ and Cr³⁺: Both Fe³⁺ and Cr³⁺ are third group cations. When treated with NH₄Cl and NH₄OH, both will precipitate as their hydroxides (Fe(OH)₃ and Cr(OH)₃). Therefore, they cannot be separated.
- Pair (ii) Cr³⁺ and Co²⁺: Cr³⁺ is a third group cation, while Co²⁺ is a fourth group cation. When treated with NH₄Cl and NH₄OH, only Cr³⁺ will precipitate as Cr(OH)₃, while Co²⁺ remains in solution. Thus, they can be separated.
- Cr³⁺ and Co²⁺
- Al³⁺ and Ba²⁺
The ions that can be separated using NH₄Cl and NH₄OH are:
- (ii) Cr³⁺ and Co²⁺
- (iv) Al³⁺ and Ba²⁺
Topper's Solved these Questions
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - MATCH THE COLUMN
3 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - ASSERTION-REASON TYPE QUESTIONS
13 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - LEVEL 3
44 Videos
- PERIODIC PROPERTIESBOOK - VK JAISWAL ENGLISHCHAPTER - PERIODIC PROPERTIESEXERCISE - MATCH THE COLUMN
11 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
2 Videos
Similar Questions
Match the following columns

View SolutionAssertion: AgNO3 reacts with KCN to form white ppt. of AgCN. This white ppt. Disappears when excess KCN is added.
Reason: AgCN decomposes to form silver-carbide and evolve N2 gas.
View SolutionA colourless inorganic compound (A) imparts a green colour to the flame. Its solution gives a white ppt. (B) with H2SO4. When heated with K2Cr2O7 and conc. H2SO4, a brown red vapour/gas (C) is formed. The gas/ vapour when passed through aqueous NaOH solution, it turns into a yellow solution (D) which forms yellow precipitate (E) with CH3COOH and (CH3COO)2Pb. with reference to above information, answe the following questions.
Q. The colourless inorganic compound (A) is:
View Solution
View Solution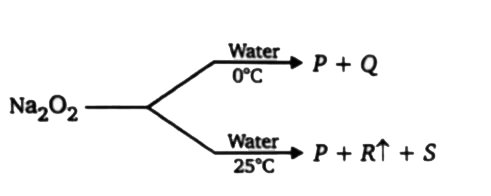
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionAssertion: The pH of NH_(4)OH remains unchanged on addition of NH_(4)Cl . Reason : Addition of NH_(4)Cl suppresses the dissociation of NH_(4)OH due to common ion effect.
View SolutionWhen NH4Cl is added to a solution of NH4OH :
View SolutionWhich of the following pairs of cations cann be separated by adding NH_(4)Cl and NH_(4)OH to the mixture?
View SolutionWhich of the following pairs of cations cannot be separated by adding NH_(4)Cl and NH_(4)OH to the mixture and then passing H_(2)S through it?
View SolutionWhich of the following pairs of cations ca be separated by adding NH_(4)Cl,NH_(4)OH and then (NH_(4))_(2)CO_(3) to the mixture?
View SolutionWhen NH_(4)Cl is not used together with NH_(4)OH in group-III reagent which of the following cation will not be precipitated?
View SolutionWhich of the following ions can be separated by using NH_(4)Cl and NH_(4)OH ?
View SolutionThe presence of NH4Cl in NH4OH solution will ............. the degree of dissociation of NH4OH.
View SolutionNH4OH+NH4Cl में अघुलनशील हाइड्रॉक्साइड है
View SolutionWhen NH_(4)Cl is added to a solution of NH_(4)OH ,
View SolutionWhich of the following pairs of cations can be separated by adding NH_(4)Cl and NH_(4)OH to the mixture?
View Solution

