Which of the following orders of acid strength is correct?
A
RCOOH>HOH>HC≡CH>ROH
B
RCOOH>RC≡CH>HOH>ROH
C
RCOOH>ROH>HOH>HC≡CH
D
RCOOH>HOH>ROH>HC≡CH
Video Solution
Text Solution
The correct Answer is:D
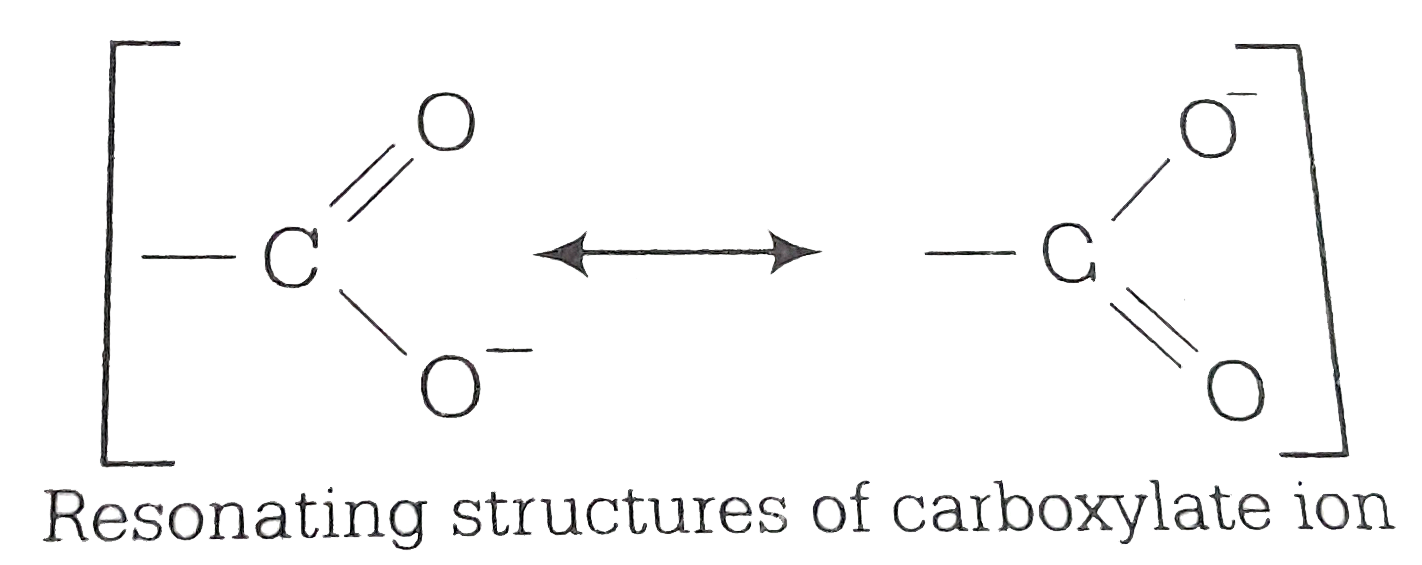
Carboxylic acid is stronger than alcohol and water because after removal of proton, carboxylate ion is stabilised by resonance. Hence, correct order of acid strength is
RCOOH gt HOH gt ROH gt HC ≡ CH
Which is based upon the rate of donation of proton or strength of base, thus order of basic strength is
¯¯¯C≡CH>R−¯¯¯O>OH−>RCOO−
|
Similar Questions
Knowledge Check
Which one of the following order of acid strength is correct ?
ARCOOH>HC≡CH>HOH>ROHBRCOOH>ROH>HOH>HC≡CHCRCOOH>HOH>ROH>HC≡CHDRCOOH>HOH>HC≡CH>ROHWhich one of the following orders of acid strength is correct?
ARCOOH>HC≡CH>HOH>ROHBRCOOH>ROH>HC≡CH>HOHCRCOOH>HOH>ROH>HC≡CHDRCOOH>HOH>HC≡CH>ROHWhich one of the following orders of acid strength is correct?
ARCOOH>HC≡CH>HOH>ROHBRCOOH>ROH>HOH>HC≡CHCRCOOH>HOH>ROH>HC≡CHDRCOOH>HOH>HC≡CH>ROH
Which of the following orders regarding acidic strength is correct
View SolutionWhich of the following orders regarding acidic strength is correct.
View SolutionWhich of the following orders of relative strength of acids is correct?
View SolutionWhich of the following decreasing order of acid strength is correct?
I. Methanoic acid II. Ethanoic acid III. Propanoic acid IV. Butanoic acid
View SolutionWhich of the following order of relative strength of acid is correct ?
View Solution
