Which of the following pairs of cations can be separated by using an NH3 solution?
Fe3+andAl3+
Al3+andZn2+
Sn2+andPb2+
Cu2+andCd2+
The correct Answer is:B
Step by step video solution for Which of the following pairs of cations can be separated by using an NH_(3) solution? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - LEVEL 3
44 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - ONE OR MORE ANSWER IS/ARE CORRECT
41 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - SUBJECTIVE PROBLEMS
3 Videos
- PERIODIC PROPERTIESBOOK - VK JAISWAL ENGLISHCHAPTER - PERIODIC PROPERTIESEXERCISE - MATCH THE COLUMN
11 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
2 Videos
Similar Questions
A colourless inorganic compound (A) imparts a green colour to the flame. Its solution gives a white ppt. (B) with H2SO4. When heated with K2Cr2O7 and conc. H2SO4, a brown red vapour/gas (C) is formed. The gas/ vapour when passed through aqueous NaOH solution, it turns into a yellow solution (D) which forms yellow precipitate (E) with CH3COOH and (CH3COO)2Pb. with reference to above information, answe the following questions.
Q. The colourless inorganic compound (A) is:
View SolutionBasic radical(s) which can not be identified by borax bead test:
View SolutionFind number of basic radicals among the following cations, which can form soluble complex on adding excess of NH4 solution.
Cd2+(aq.),Pb2+(aq.),Ni2+(aq.),Mn2+(aq.),Zn2+(aq.),Ag+(aq.),Hg2+(aq.),Fe3+(aq.),Mg2+(aq.)
View Solution
View Solution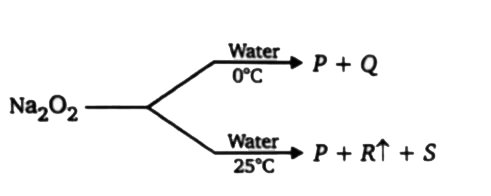
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionWhich of the following pairs of cations cannot be separated by using an NH3 solution?
View SolutionWhich of the following pair of cations cann be separated by excess NaOH solution?
View SolutionWhich of the following pairs of cations cannot be separated by using an NH_(3) solution?
View SolutionWhich of the following pairs of cations can be separted by using on adding NaOH solution?
View SolutionWhich of the following pairs of cations ca be separated by adding NH_(4)Cl,NH_(4)OH and then (NH_(4))_(2)CO_(3) to the mixture?
View SolutionWhich of the following pairs of cations can be separated by using an NH_(3) solution?
View SolutionWhich of the following pairs of cations cannot be separate by using an NaOH solution?
View SolutionWhich of the following pairs of cations can be separated by using an NaOH solution?
View SolutionWhich of the following pairs of cations can be separated by adding NH_(4)Cl and NH_(4)OH to the mixture?
View SolutionWhich of the following mixtures can be separated by using an NH_(3) solution?
View SolutionWhich of the following mixtures of ions in solution can be separated by using an NH_(3) solution
View Solution

