Which of the following pairs of ions are isoelectronic and isostructural?
A
CO2−3,SO2−3
B
ClO−3,CO2−3
C
SO2−3,NO−3
D
ClO−3,SO2−3
Video Solution
Text Solution
The correct Answer is:D
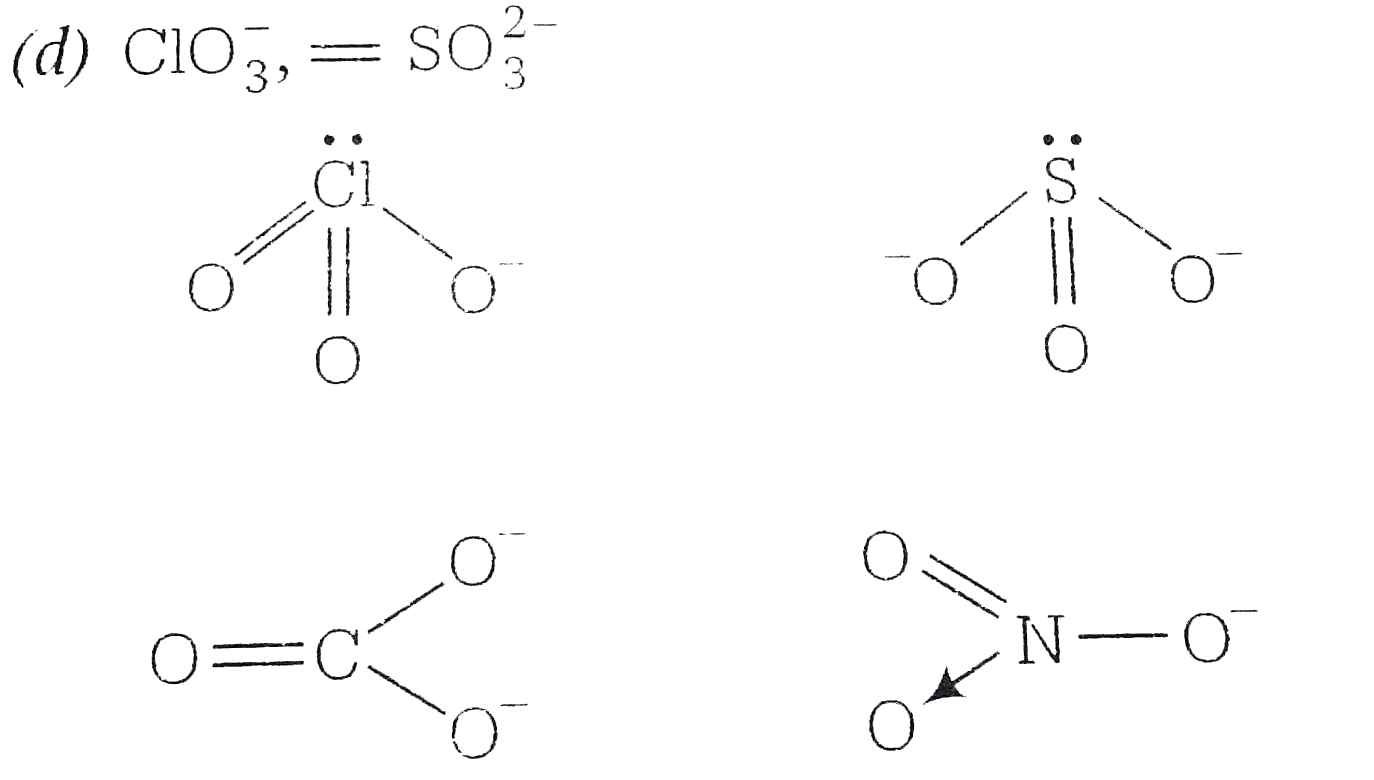
Number of electrons
CO2−3=6+2+24=32
SO2−3 =16+2+24=42
ClO−3=4+24+1=42
CO2−3=6+24+2=32
NO−3=7+2+24=33
Hence, ClO−3 and SO2−3 are isoelectronic and are pyramidal in shape.
|
Similar Questions
Which of the following pairs of ions is isoelectronic and isostructural?
View SolutionWhich of the following pairs of ions are isoelectronic and also isostructural ?
View SolutionWhich of the following pairs of ions isoelectronic and isostructural ?
View SolutionWhich of the following pairs of ions is isoelectronic and isostructural
View SolutionWhich of the following pairs of ions is isoelectronic and isostructural
View Solutionनिम्न में से कौन सा आयनों का युग्म समइलेक्ट्रॉनी एवं समसंरचनात्मक है?
View Solutionनिम्न में से आयनों का कौन-सा युग्म सम्इलेक्ट्रॉनिक एवं समसंरचनात्मक है?
View SolutionWhich of the following pairs of ions are isoelectronic and also isostructural ?
View Solution
