Why transition element show coloured Ions ? Explain. Solution in Punjabi
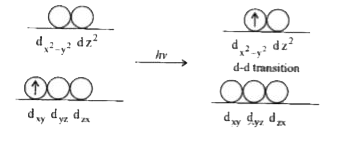
The colour of the compounds of transition metals may be attributed due to the presence of incomplete (n−1)d subshell. In the case of compounds of transition metals, the energies of five d-orbitals in the same subshell do not remain equal. Under the Influence of approaching ions(ligands) towards the central ion, the d-orbitals of the central metal split into different energy levels. This Phenomenon is called Crystal field splitting. In the., case the transition metal ions, the electrons can be easily promoted from one energy level to another in the same d-subshell. These are called d-d transition.
Topper's Solved these Questions
- D & F BLOCK ELEMENTS BOOK - ACCURATE PUBLICATIONCHAPTER - D & F BLOCK ELEMENTS EXERCISE - 1 OR 2 MARK QUESTIONS
40 Videos
- COORDINATION COMPOUNDS BOOK - ACCURATE PUBLICATIONCHAPTER - COORDINATION COMPOUNDS EXERCISE - 2 MARKS QUESTIONS
23 Videos
- ELECTRO CHEMISTRYBOOK - ACCURATE PUBLICATIONCHAPTER - ELECTRO CHEMISTRYEXERCISE - Numbericals Practice
44 Videos
Similar Questions
Name the elements which lie between s-and p-block in the long form of the periodic table ?
View SolutionWhat is the difference between a complex salt and a double salt?
View SolutionGive cell is [Ni∣∣Ni2+∣∣∣∣Cu2+∣∣Cu] (0.01m) (0.1m)
Ecell=0.59V
Write Nernst equation and find E∘N2+i/Nl of cell. (At anode) E∘Cu2+/Cu=0.39V
View SolutionScandium forms no coloured ions, yet it is regarded as a transition elements. Explain why?
View Solutionसंक्रमण तत्वों द्वारा रंगीन आयन की व्याख्या कीजिए ।
View SolutionScandium forms no coloured ions, yet it is regarded as a transition elements. Explain why?
View SolutionScandium is considered to be a transition element though it does not form any coloured ion. Explain.
View Solution(a) Define transition elements. (b) Give general electronic configuration of f-block elements.(c ) Most of transition elements are coloured, why? (d ) Transition elements show variable oxidation states, why?
View SolutionWhy are the compound of transition elements coloured?
View SolutionExplain why transition metal ions are usually coloured.
View SolutionTransition metal ions are generally coloured. Why?
View SolutionTransitional metal ions shows colour due to
View SolutionScandium forms no coloured ions, yet it is regarded as a transition elements. Explain why?
View SolutionThe elements which form coloured ions and show paramagnetism are: Alkali metals , Alkaline earth metals , Halogens , Transition elements.
View SolutionExplain , why transition metal ions are usually coloured ?
View SolutionExplain why most of transition elements from coloured salts.
View Solution

