Write the resonance structures of (1)CH3COOӨ and (2)CH2=CH−CHO. Indicate the relative stability of the contributing structures.
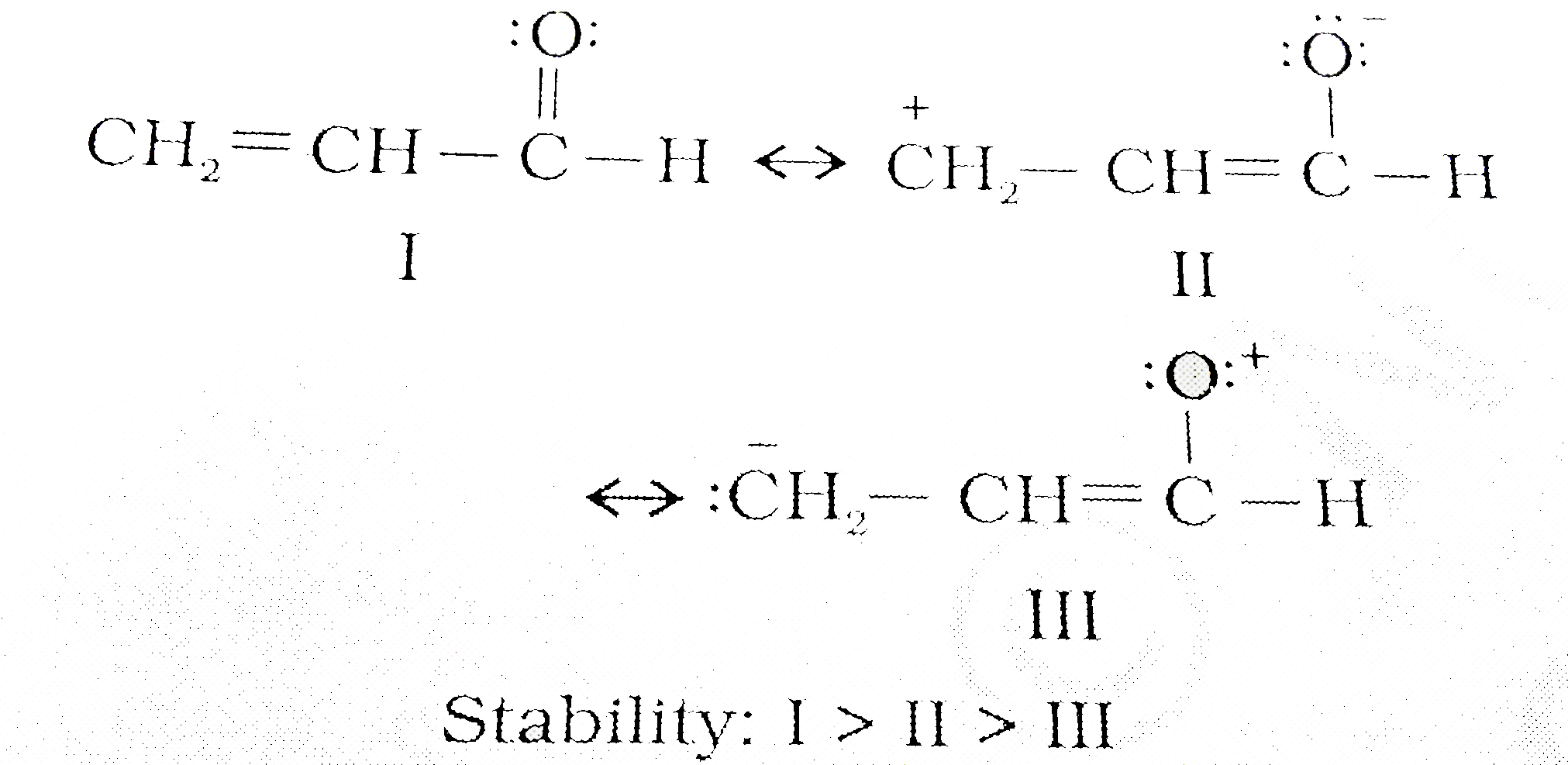
[I: Most stable, more number of covalent bonds, each carbon and oxygen atom has an octet and no separation of opposite charge II: negative charge on more electronegative atom and positive charge on more electropositive atom, III: does not contribute as oxygen has positive charge and carbon has negative charge, hence least stable].
Topper's Solved these Questions
- ORGANIC CHEMISTRY- SOME BASIC PRINCIPLES AND TECHNIQUESBook:NCERTChapter:ORGANIC CHEMISTRY- SOME BASIC PRINCIPLES AND TECHNIQUESExercise:EXERCISE
Explore 40 Videos
- HYDROGENBook:NCERTChapter:HYDROGENExercise:EXERCISE
Explore 36 Videos
- REDOX REACTIONSBook:NCERTChapter:REDOX REACTIONSExercise:EXERCISE
Explore 30 Videos
Similar Questions
Given the hybridistaiton state of each carbon in the following compounds :
I. CH2=C=O II. CH3−CH=CH2
III. (CH3)2CO IV. CH2=CH=C≡N
v. C6H6
View SolutionJustify the position of hydrogen in the periodic table on the basis of its electronic configuration.
View SolutionAssign oxidation number to the underlined elements in each of the following species:
a.NaH2PO4
b. NaHS––O4
c. H4P2–––O7
d. K2Mn––––O4
e. Ca–––O2
f. NaB––H4
g. H2S2–––O7
h. KAl(S––O4)2.12H2O
View SolutionWrite the resonance structures of (1) CH_3 COO^(Ө) and (2)CH2=CH−CHO. Indicate the relative stability of the contributing structures.
View SolutionThe most stable resonating structure of CH_3-underset(..)ddotO-CH=CH_2 is
View SolutionWrite resonating structures for CH2=CHCHO. Indicate relative stability of the contributing structures :
View Solutionयौगिकों के IUPAC नाम लिखिए -
विविध उदाहरण संरचना सूत्र (IUPAC नाम) ⎛⎜⎝CH3−CH2−C∣CHOH−CH2−CH3⎞⎟⎠
View SolutionCH2=CH−CHO की अनुनाद - संरचनाएँ लिखें तथा विभिन्न अनुनाद - संरचनाओं के आपेक्षिक स्थायित्व को दर्शाएँ |
View SolutionWrite resonance structures of CH2=CH–CHO. Indicate relative stability of the contributing structures.
View Solutionక్రింది నిర్మాణాలఉ IUPAC నమలను వ్రాయండి. CH3−CH2−CH2−CH=CH2
View SolutionWrite resonance structures of the following showing the movement of electrons by curved arrows :
(i) Acetate ion, CH3COO−
(ii) CH3CH=CH−C+H2
View SolutionWrite resonating structures for CH_2 = CHCHO . Indicate relative stability of the contributing structures :
View SolutionWrite resonating structures of CH3COO− ion.
View SolutionCH_2=CH-CH=O Write resonant structures and show the relative stability of various resonance structures.
View SolutionWrite the resonance structures of (1) CH_3 COO^(Ө) and (2) CH_2 = CH - CHO . Indicate the relative stability of the contributing structures.
View SolutionWrite the structures of the product of the following reaction
Ch3−CH2−C∣CH3H−CHONaBH4−−−−→
View Solution
