A container of volume 4V0 made of a perfectly non− conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non− conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non− conducting piston free to move as shown . Section A contains 2mol of a gas while the section B and C contain 1mol each of the same gas (γ=15.) at pressure P0 . The heater in left part is switched on till the final pressure in section C becomes 125/27P0. Calculate
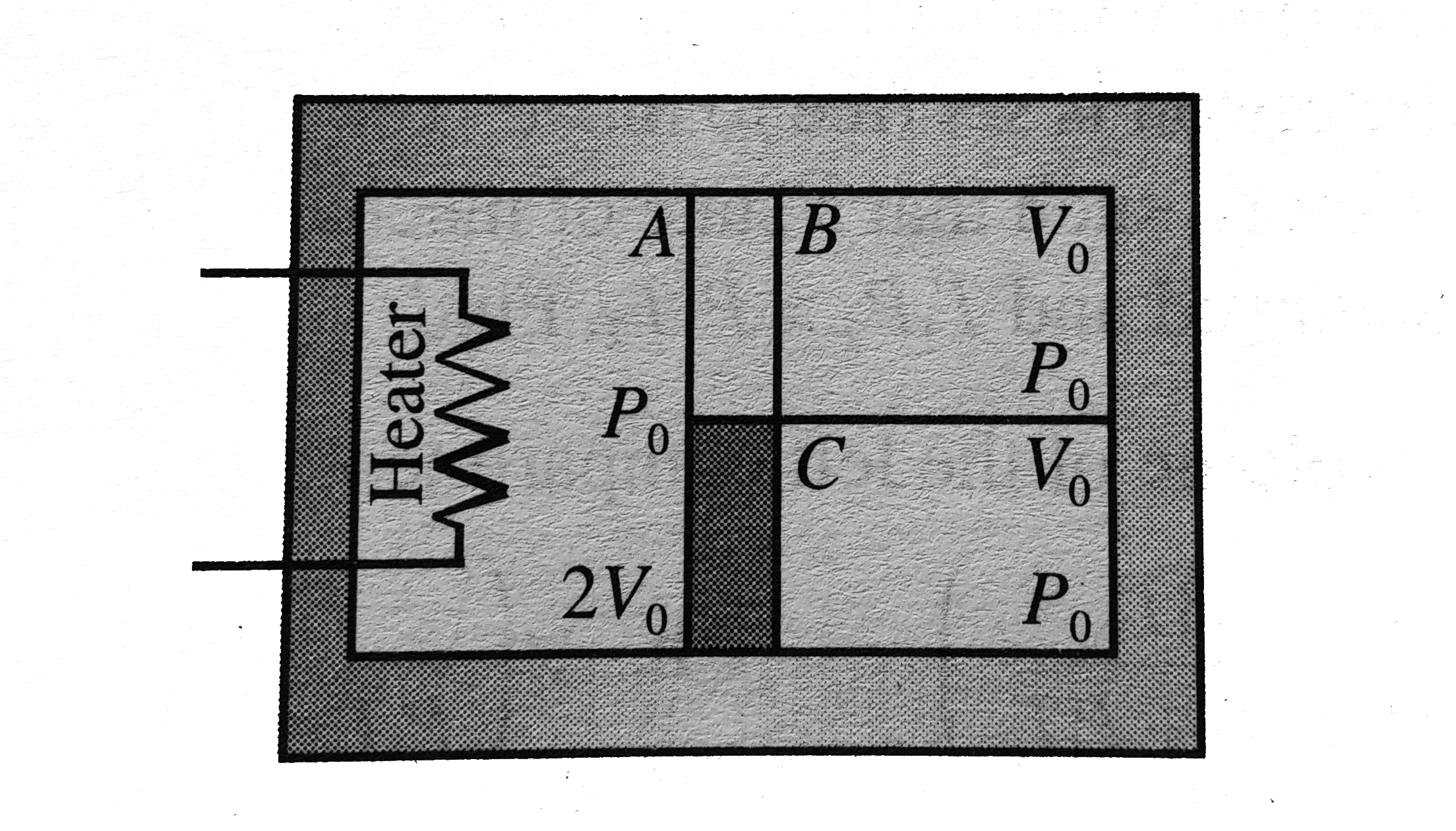
The heat supplied by the heater.
3689P0V0
1135P0V0
3169P0V0
4058P0V0
The correct Answer is:A
For 'C',P0Vγ0=12527P0VγC
VC=V0(925)
VB=2V0−9V025=(50−9)25V0
VB=4125V0
T0A=T0B=P0V0R
TC=12527P0×925V0R=5P0V03R
TB=12527P0×4125V0R=20527P0V03R=TA
Q=nACV(TA−T0)+nBCVΔTB+nCCVΔTC
Q=2.2R(20527−1)P0V0R+1.2R(20527−1)P0V0R+1.2R[53P0V0R−P0V0R]
=2P0V0[178×227+17827+23]=3689P0V0
Similar Questions
A non-conducting cylinder having volume 2V0 is partitioned by a fixed non conducting wall in two equal part. Partition is attached with a valve. Right side of the partition is a vacuum and left part is filled with a gas having pressure and temperature P0 and T0 respectively. If valve is opened find the final pressure and temperature of the two parts.
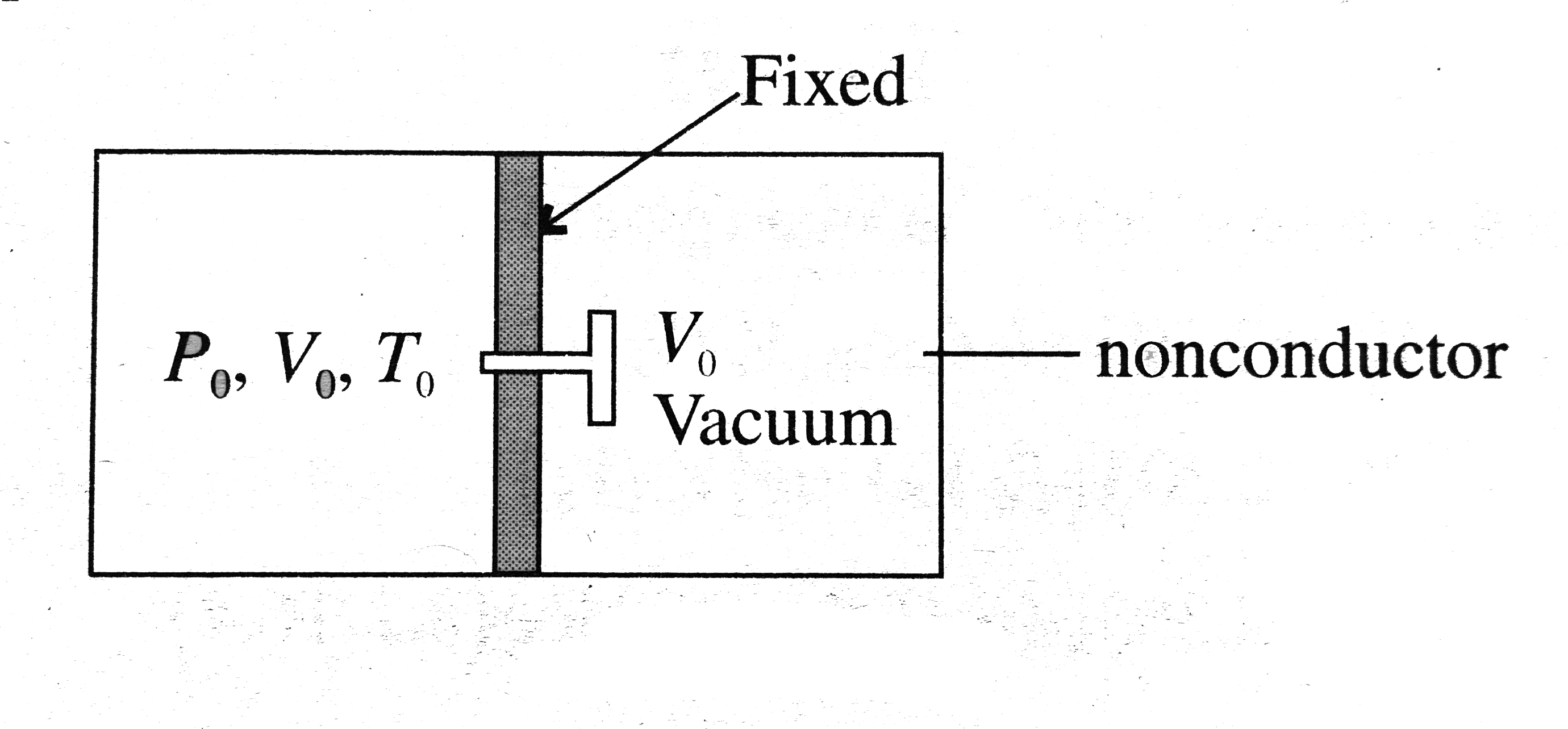
View SolutionA container of volume 4V0 made of a perfectly non− conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non− conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non− conducting piston free to move as shown . SectionA constains 2mol of a gas while the section B and C contain 1mol each of the same gas (γ=15.) at pressure P0 . The heater in left part is switched on till the final pressure in section C becomes 125/27P0. Calculate
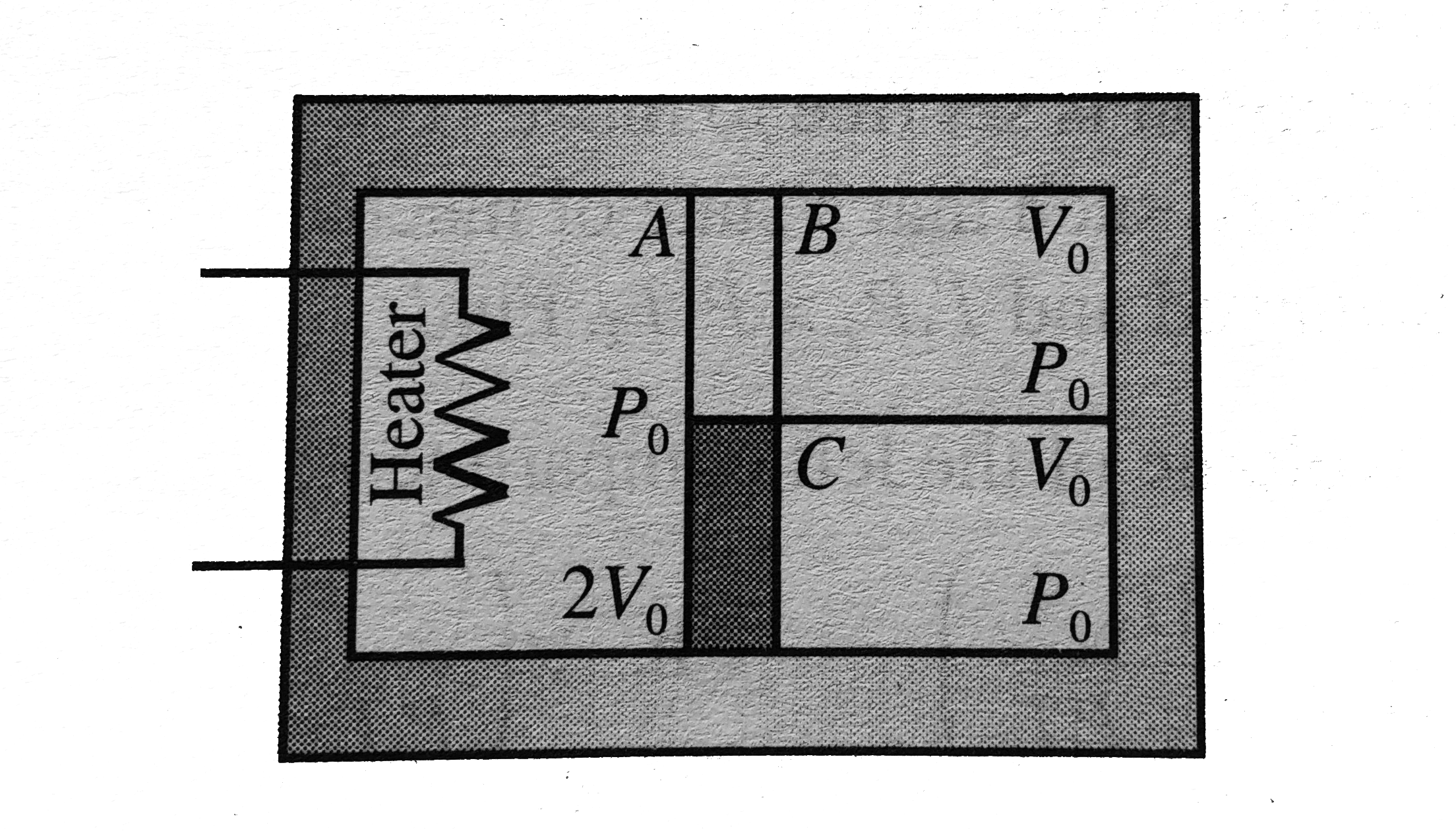
Final temperature in part C.
View SolutionA container of volume 4V_(0) made of a perfectly non - conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non - conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non - conducting piston free to move as shown . Section A constains 2 mol of a gas while the section B and C contain 1 mol each of the same gas ( gamma = 15. ) at pressure P_(0) . The heater in left part is switched on till the final pressure in section C becomes 125//27 P_(0) . Calculate The heat supplied by the heater.
View SolutionA cylindrical container having non-conducting walls is partitioned in two equal parts such that the volume of the each parts is eqaul to V_(0) A movable non-conducting piston is kept between the two parts. Gas on left is slowly heated so that the gas on right is compressed upto volume V_(0)//8 . Find pressure and temperature on both sides if initial pressure and temperature, were P_(0) and T_(0) respectively. Also find heat given by the heater to the gas (number of moles in each parts is n)
View SolutionComprehension-2 A mono atomic ideal gas is filled in a non conducting container. The gas can be compressed by a movable non conducting piston. The gas is compressed slowly to 12.5% of its initial volume. The percentage increases in the temperature of the gas is
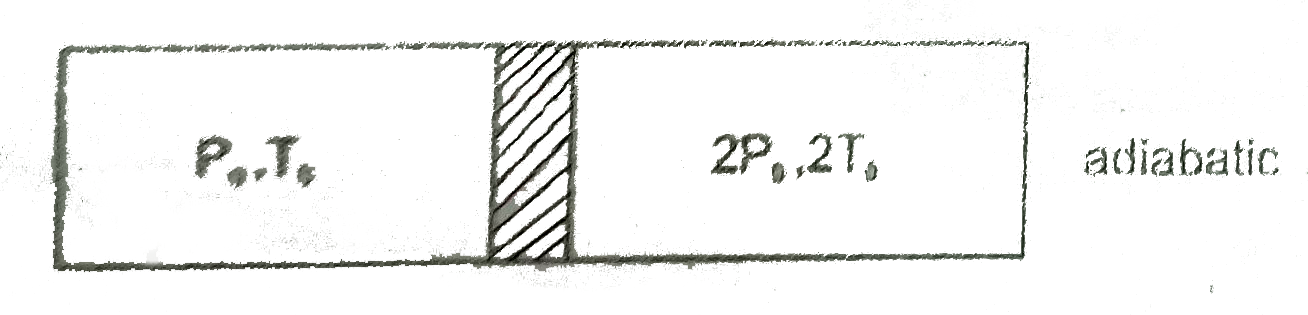
View SolutionA cylindrical tube with adiabatic walls having volume 2V0contains an ideal monoatomic gas as shown in figure. The tube is divided into two equal parts by a fixed super conducting wall. Initially, the pressure and the temperature are P0,T0 on the left and 2P0,2T0 on the rigid. When system is left for sufficient amount of time the temperature on both sides becomes equal
View SolutionA container of volume 4V0 made of a perfectly non− conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non− conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non− conducting piston free to move as shown . SectionA constains 2mol of a gas while the section B and C contain 1mol each of the same gas (γ=15.) at pressure P0 . The heater in left part is switched on till the final pressure in section C becomes 125/27P0. Calculate

The heat supplied by the heater.
View SolutionA container of volume 4V0 made of a perfectly non− conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non− conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non− conducting piston free to move as shown . SectionA constains 2mol of a gas while the section B and C contain 1mol each of the same gas (γ=15.) at pressure P0 . The heater in left part is switched on till the final pressure in section C becomes 125/27P0. Calculate

Final temperature in part C.
View Solution
