A gas has been subjected to isochoric-isobaric processes 1-2-3-4-1 see Fig. Plot this process on T - V and P - T diagrams.
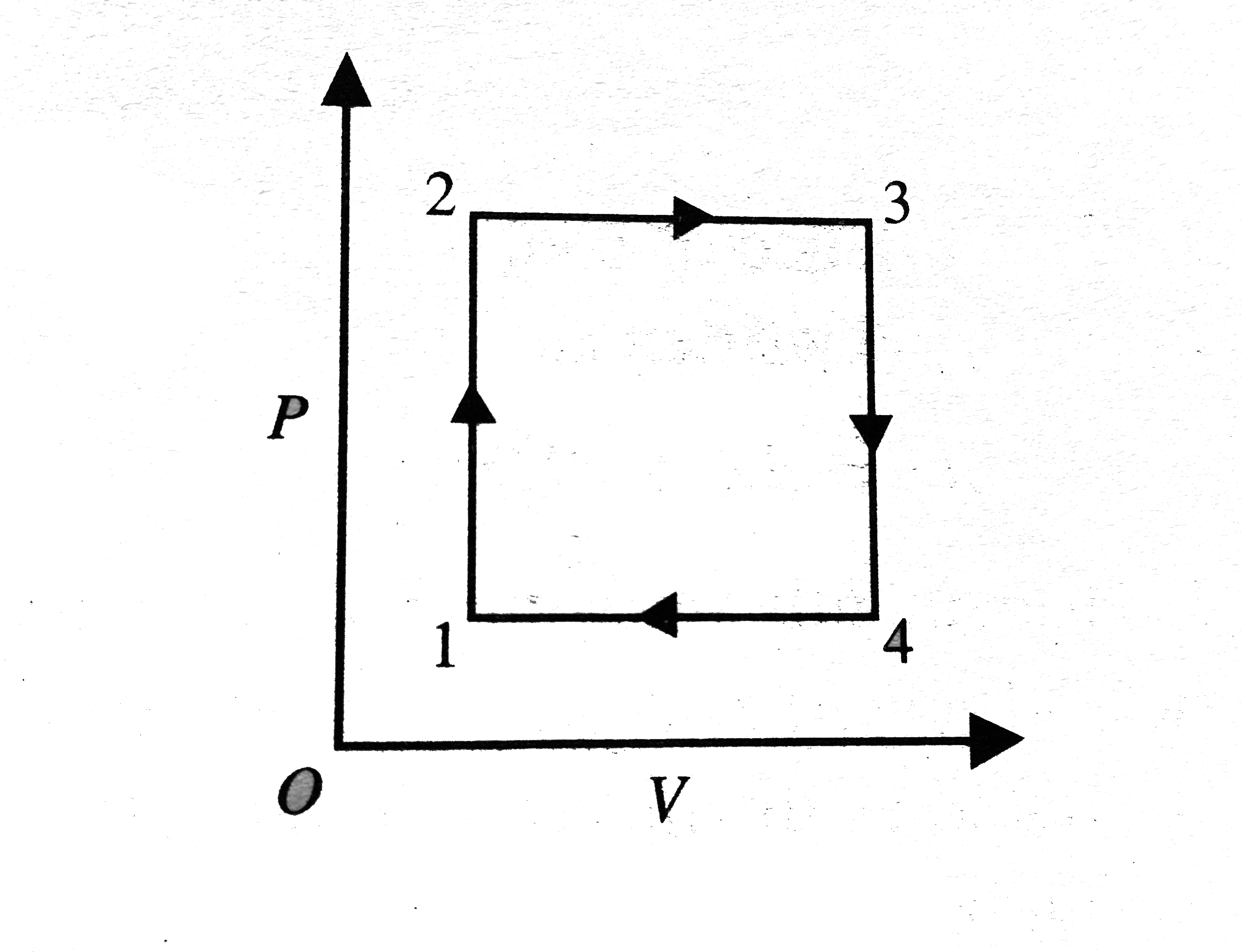
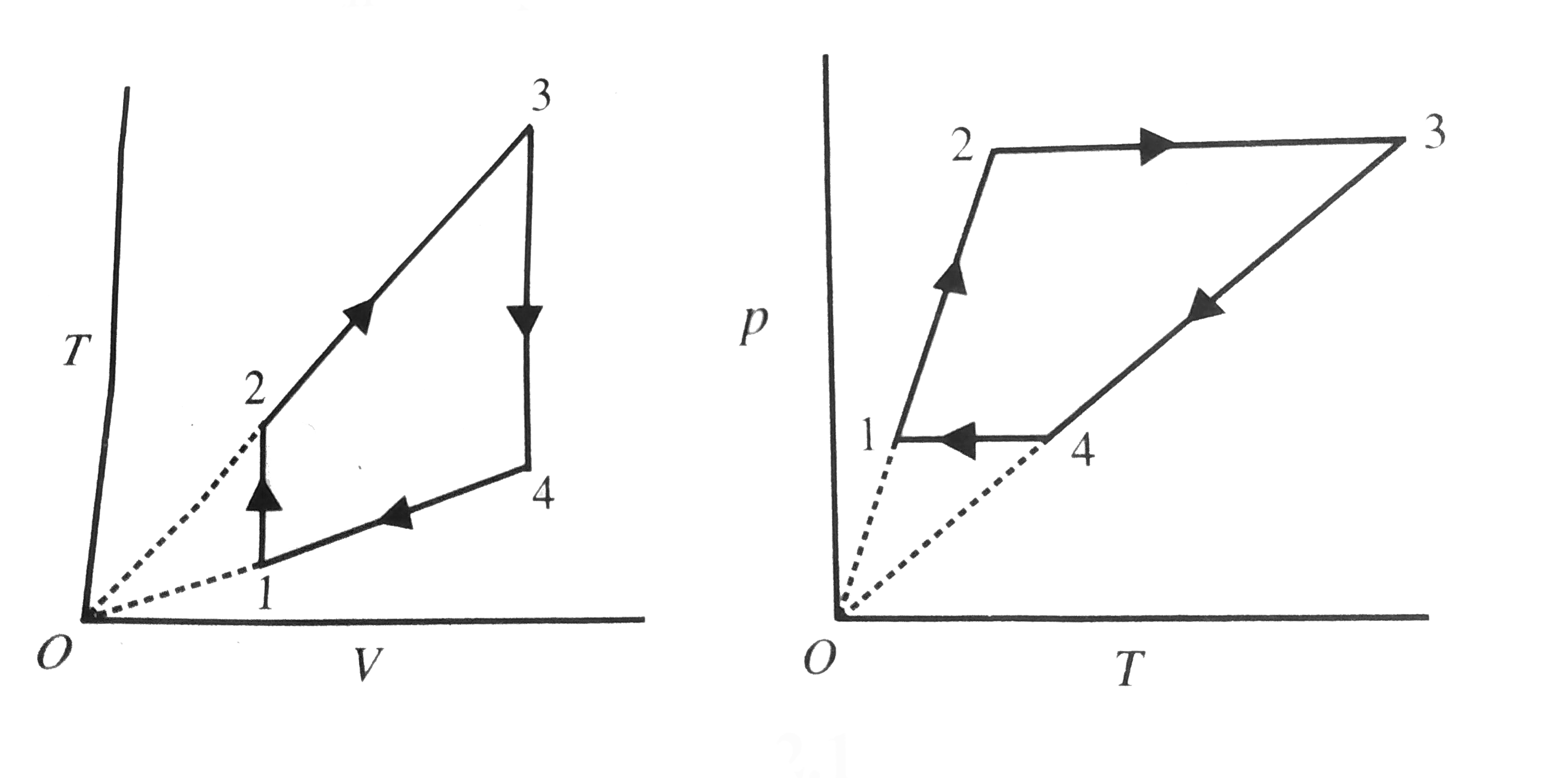
The plot is shown in Fig.
Explanation: Since 1−2,V remains constant, the plot of T against V is a straight line parallel to the T−aξs.
For an ideal gas pV=RT. So when p remains constant, V∞T. Hence the plot of T against V is a straight line passing through the origing. The same is repected fro 3−4 and 4−1. The plot of p against T is shown in the figure and can be explained similarly.
টপার এই প্রশ্নগুলো সমাধান করেছে
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Exercise 2.2
Explore 28 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Subjective
Explore 22 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Solved Examples
Explore 14 Videos
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASESExercise:Compression
Explore 2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBook:CENGAGE PHYSICS ENGLISHChapter:LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONExercise:Single correct anwer type
Explore 14 Videos
সম্পর্কিত প্রশ্ন
Explain why the temperature of a gas drops in an adiabatic expansion, on basis of the kinetic theory of gases,
View SolutionFive grams of helium having rms speed of molecules 1000m/s and 24g of oxygen having rms speed of 1000m/s are introduced into a thermally isolated vessel. Find the rms speeds of helium and oxygen individually when thermal equilibrium is attained. Neglect the heat capacity of vessel.
View SolutionA vessel containing 1 g of oxygen at a pressure of 10 atm a temperature of 47∘C. It is found that because of a leak, the pressure drops to 5/8th of its original value and the temperature falls to 27∘C. Find the volume of the vessel and the mass of oxygen that is leaked out.
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
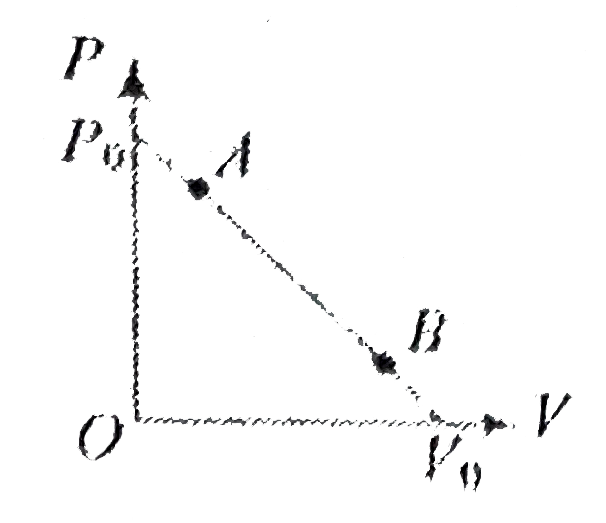
The absolute temperature T versus volume V for the given process is
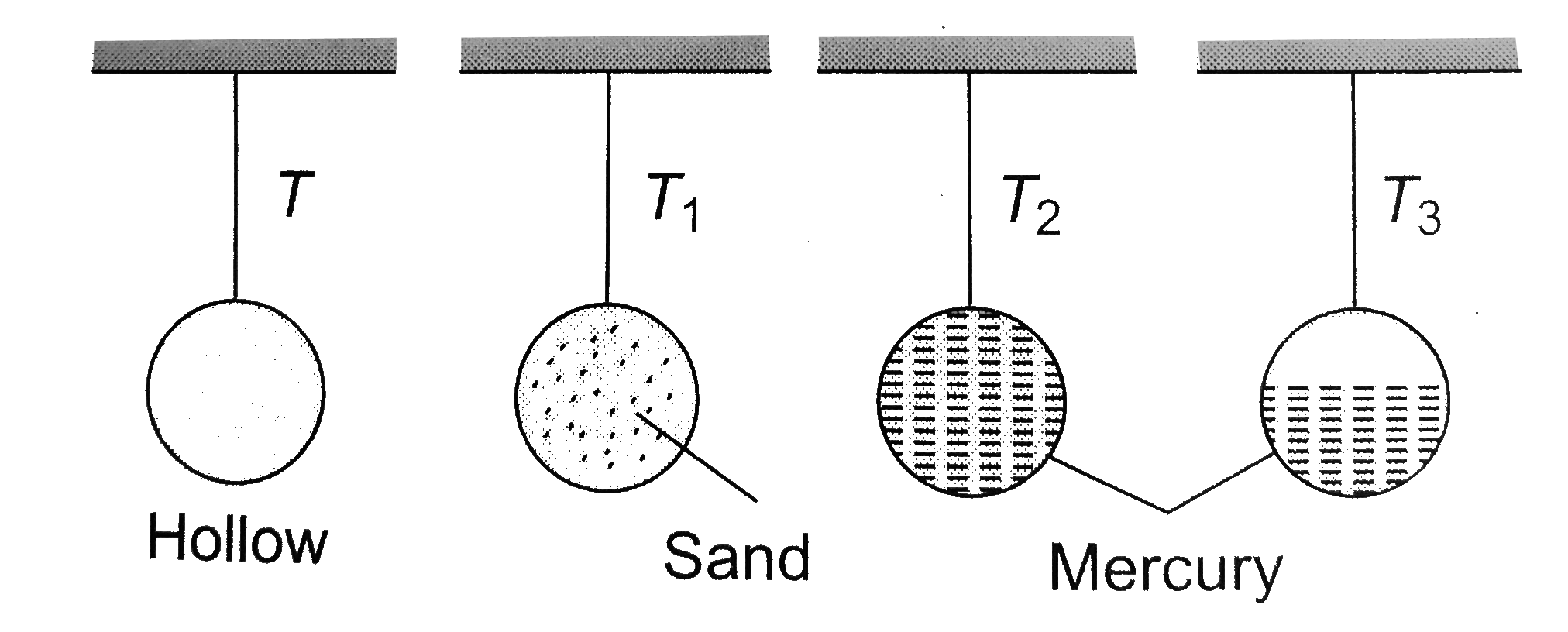
View SolutionThe period of a simple pendulum whose bob is hollow metallic sphere is T. The period is T1 when the bob is filled with sand, T2 where it is filled with mercury and T3 when it is half filled with mercury Which of the following is true?
View SolutionStraight line on (p - T) graph for an ideal gas represents isochoric process.
If p∝T,V=constant.
View SolutionA cyclic process 1-2-3-4-1 is depicted on V-T diagram. The p-T and p-V diagrams for this cyclic process are given below. Select the correct choices (more than one options is/are correct)
View SolutionA gas has been subjected to isochoric-isobaric processes 1-2-3-4-1 see Fig. Plot this process on T - V and P - T diagrams.
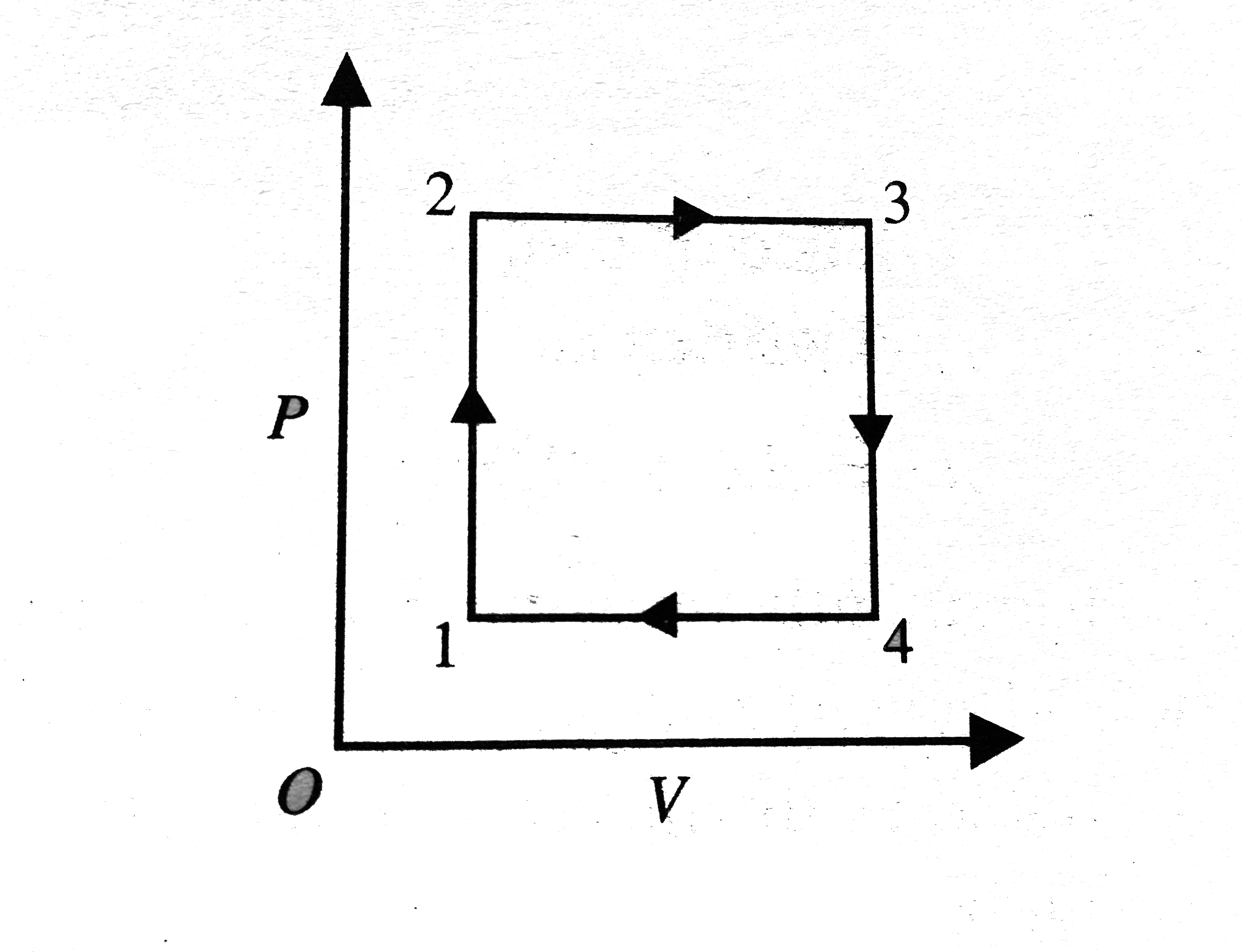
View SolutionA gas has been subjected to an isothermal-isochromic cycle 1-2-3-4-1 see Fig. Represent the same cycle on P - V and P - T diagrams.
View SolutionWhat is the nature of P-V diagram for isobaric and isochoric processes?
View SolutionDraw the approximate plots of isochoric, isobaric, isothermal, and adiabatic processes for the case of an ideal gas, using the following variables : (a) p, T : (b) V , T .
View SolutionA gas has been subjected to an isochoric and isobaric cycle. Plot of the graph of this cycle in the pressure-density, V-T and P-T co-ordinates are given below. Choose the correct plots.
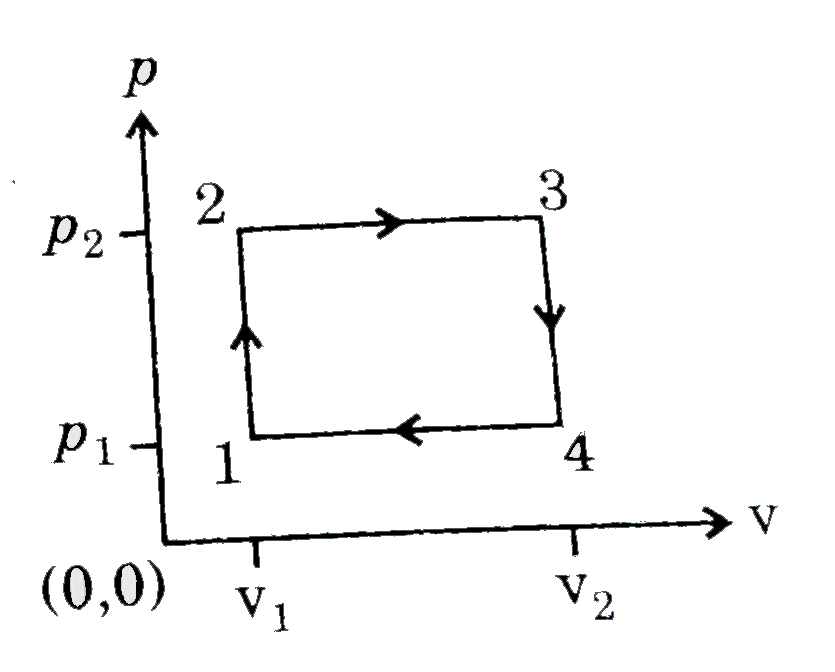
View SolutionThe plots between P and V which represent isochoric and isobaric process respectively:
View SolutionA gas has been subjected to a cycle of isochoric, isothermal process 1-2-3-4-1 as shown in the figure. The graph for this cycle on P-T diagram is
View SolutionDefine the following terms: (a) Isothermal process (b) adiabatic process (c) isobaric proces (d) isochoric process.
View Solution
