A gas kept in a container of finite conductivity is suddenly compressed . The process
may be very nearly adiabatic
may be nearly isothermal
must be very nearly adiabatic
must be very nearly isothermal
The correct Answer is:A, B
When the gas is suddenly compressed, the process may be very nearly adiabatc.As container has finite conductivity, heat developed will flow out in some time. The process may be very nearly isothermal.
Similar Questions
Two cylinders A and B of equal capacity are connected to each other vis a stop cock. A contains an ideal gas at standard temperature and pressure. B is completely evacuated. The sto cock is suddenly opened. The process is:
View SolutionA Gas is enclosed in a metallic container of volume Vand its initial pressure is p. It is slowly compressed to a volume V/2 and then suddenly compressed to V/4. Find the final pressure of the gas. From the initial state the gas is suddenly compressed to V/2 and then slowly compressed to V/4, what will be the final pressure now.
View Solution
Knowledge Check
A gas is a metallic cylinder is suddenly compressed by a piston which is maintained at the same position with the passage of time the pressure of air
AincreasesBdecreaseCremain the sameDnone of theseA sample of a gas is kept in a closed container and temperature is increased. Which of the following is true?
APressure is increased because momentum transferred per collision to wall is increased.BPressure is decreasedCPressure is increased because frequency of collision is decreasedDBoth (A) and (C) are correct400 cc volume of a gas having γ=5/2 is suddenly compressed to 100 cc. If the initial pressure is P, then the final pressure will be
AP/32B8PC32PD16 P
When a gas is suddenly compressed, its temperature rises. Why?
View SolutionTwo moles of a diatomic gas at 300 K are kept in a nonconducting container enclosed by a piston . Gas is now compressed to increase the temperature from 300 K to 400 K. Find word done by the gas

View SolutionHelium at 27∘C has a volume of 8 litres. It is suddenly compressed to a volume of 1 litre. The temperature of the gas will be [γ=5/3]
View SolutionTwo moles of a monoatomic gas at 300K are kept in a non conducting container enclosed by a piston . Gas is now compressed to increase the temperature from 300K to 400K. Find work done by the gaS (R=253J/mol−K)
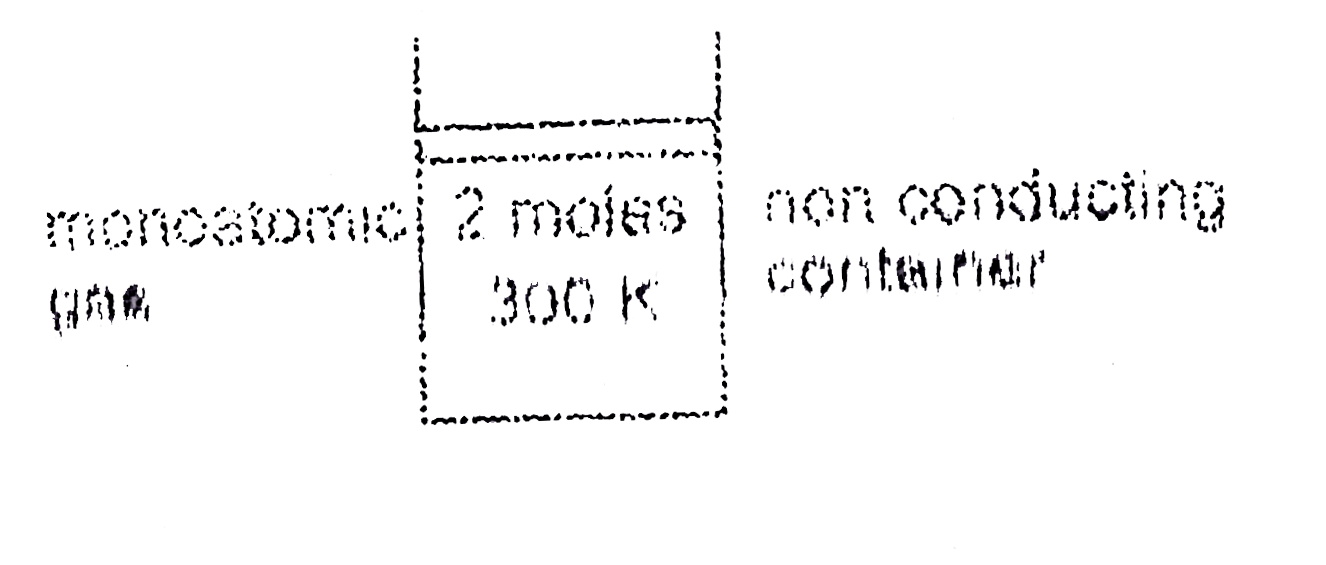
View SolutionA gas of γ=1.4 and initial temperature 0∘C is suddenly compressed to 1/5th its original volume. Calculate the rise in temperature.
View Solution
