A monatomic idea gas of 2mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are VB/VA=2 and VD/VA=4. If the temperature TA at A is 27∘C, and gas constant is R. Calculate heat absorbed or released by the gas in process D→A.
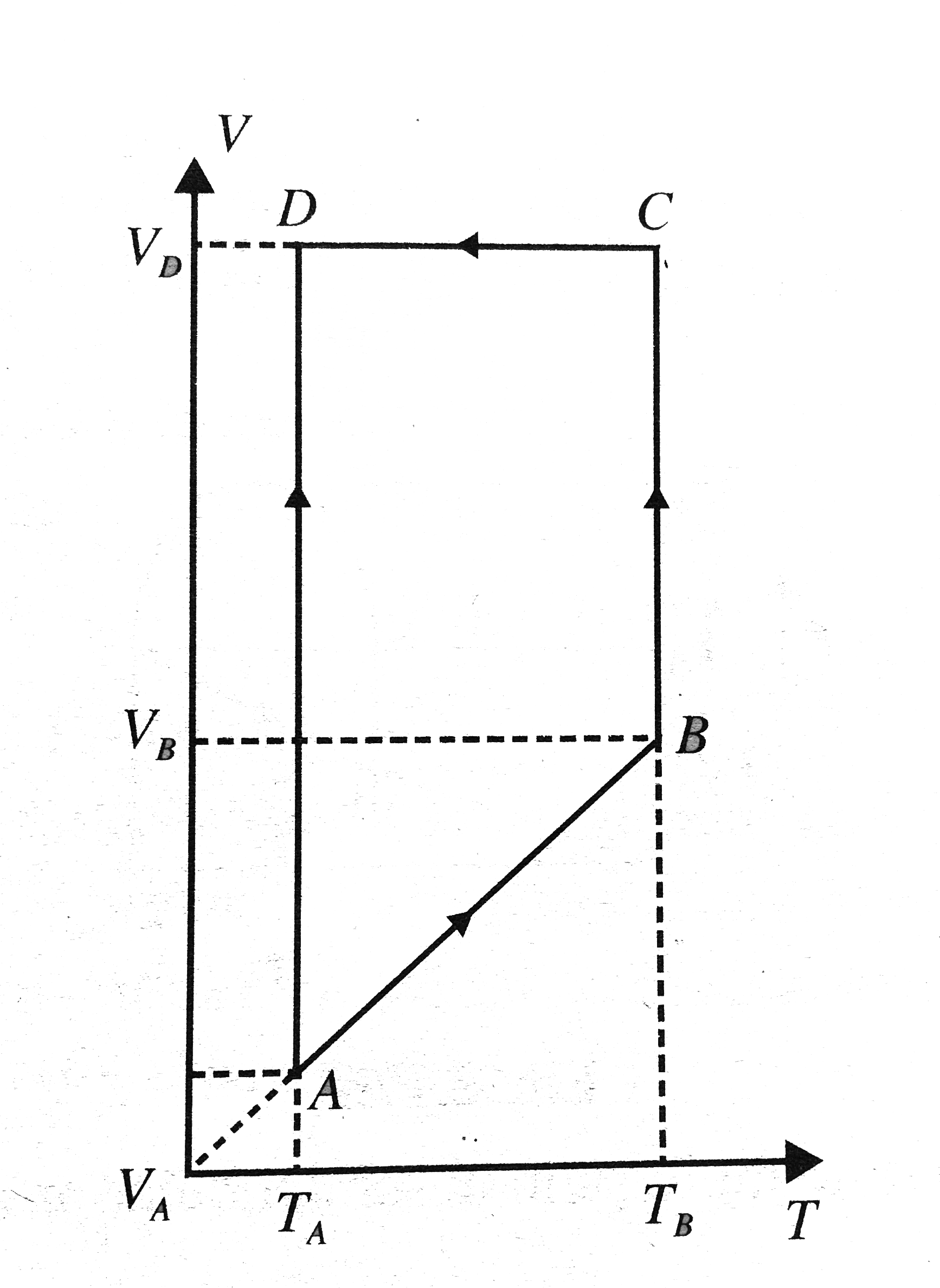
1500, added
1200Rln(2), added
900R, rejected
1200Rln(2), rejected
The correct Answer is:D
Gas is monatomic, hence
Cv=32R and Cp=52R
Number of moles, n=2
TA=27∘C=300K
Process A→B is a striaght line passing through origin, hence it is an isobaric process.
VATA=VBTB
TB=(VBVA)TA
=2×300K=600K ltbr> Porcess A→B is isobaric.
Qp=nCp(TB−TA)
Qp=2(52R)(600−300)=1500R
Heat is added to the system.
ProcessB→C is isothermal.
From the first law of thermodynamics.
QBC=WBC
( as ΔU=0)
W=nRTB1n(VCVB)
=(2)(R)(600)1n(4VA2VA)
=(1200R)1n(2)
Heat is added to the system.
Process C→D is isochoric.
From the first law of thermodynamics,
QCD=nCv(TD−TC)=n(32R)(TA−TB)
=2(32R)(300−600)=−900R
Negative sign implies that heat is rejected by the system.
Process D→A is isothermal.
QDA=WDA=nRT01n(VAVD)
=(2)(R)3001n(VA4VA)=600R1n(14)
Negative sign implies that heat is rejected by the system.
In a cyclic process, ΔU=0
Qnet=Wnet
Wnet=QAB+QBC+QCD+QDA
=1500R+8.316R−900R−831.6R=600R
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Interger
Explore 11 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Assertion-Reasoning
Explore 6 Videos
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASESExercise:Compression
Explore 2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBook:CENGAGE PHYSICS ENGLISHChapter:LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONExercise:Single correct anwer type
Explore 14 Videos
Similar Questions
8g of oxygen, 14 g of nitrogen and 22 g carbon dioxide are mixed in an encloser of volume 10 litre and temperature 27∘C. Calculate the pressure exerted by the mixture , R=8.3Jmole−1K−1, Molecular weight of oxygen , nitrogen and carbon 32 , 28 and 44 respectively.
View SolutionStatement I: A quasi -static process is so called bacause it is a sudden and large change of the system.
Statement II: An adiabatic process is not quasi- static because it is a sudden and large change of the system.
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
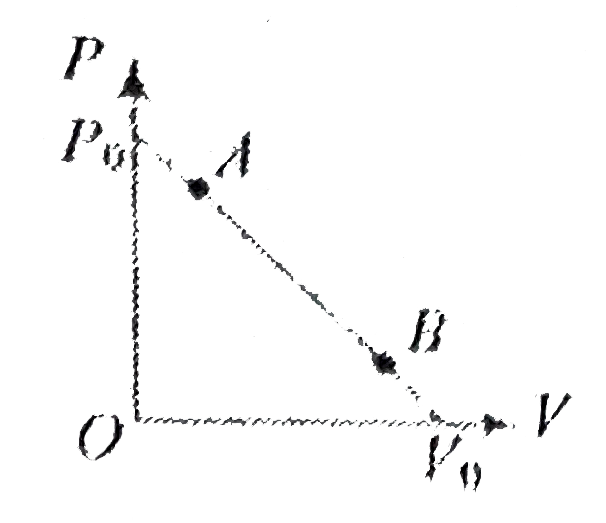
The absolute temperature T versus volume V for the given process is
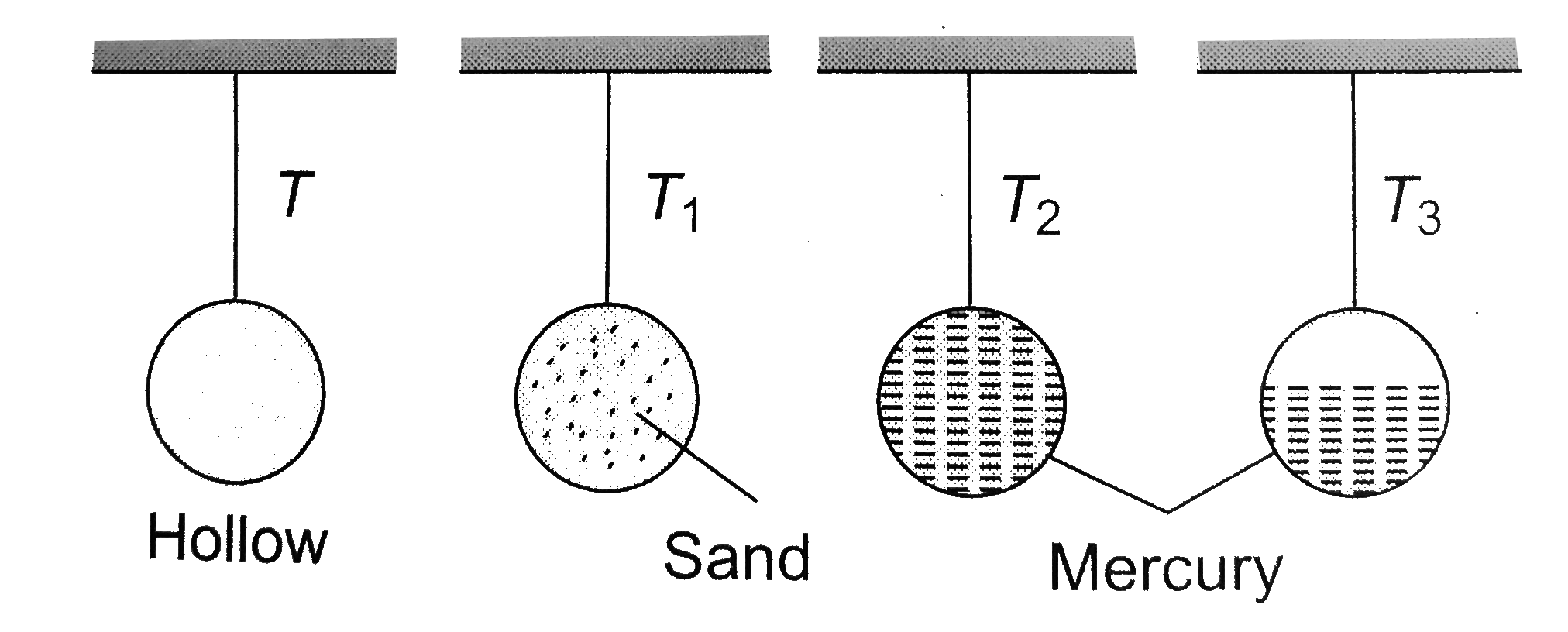
View SolutionThe period of a simple pendulum whose bob is hollow metallic sphere is T. The period is T1 when the bob is filled with sand, T2 where it is filled with mercury and T3 when it is half filled with mercury Which of the following is true?
View SolutionA monatomic idea gas of 2mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are VB/VA=2 and VD/VA=4. If the temperature TA at A is 27∘C, and gas constant is R. Calculate.
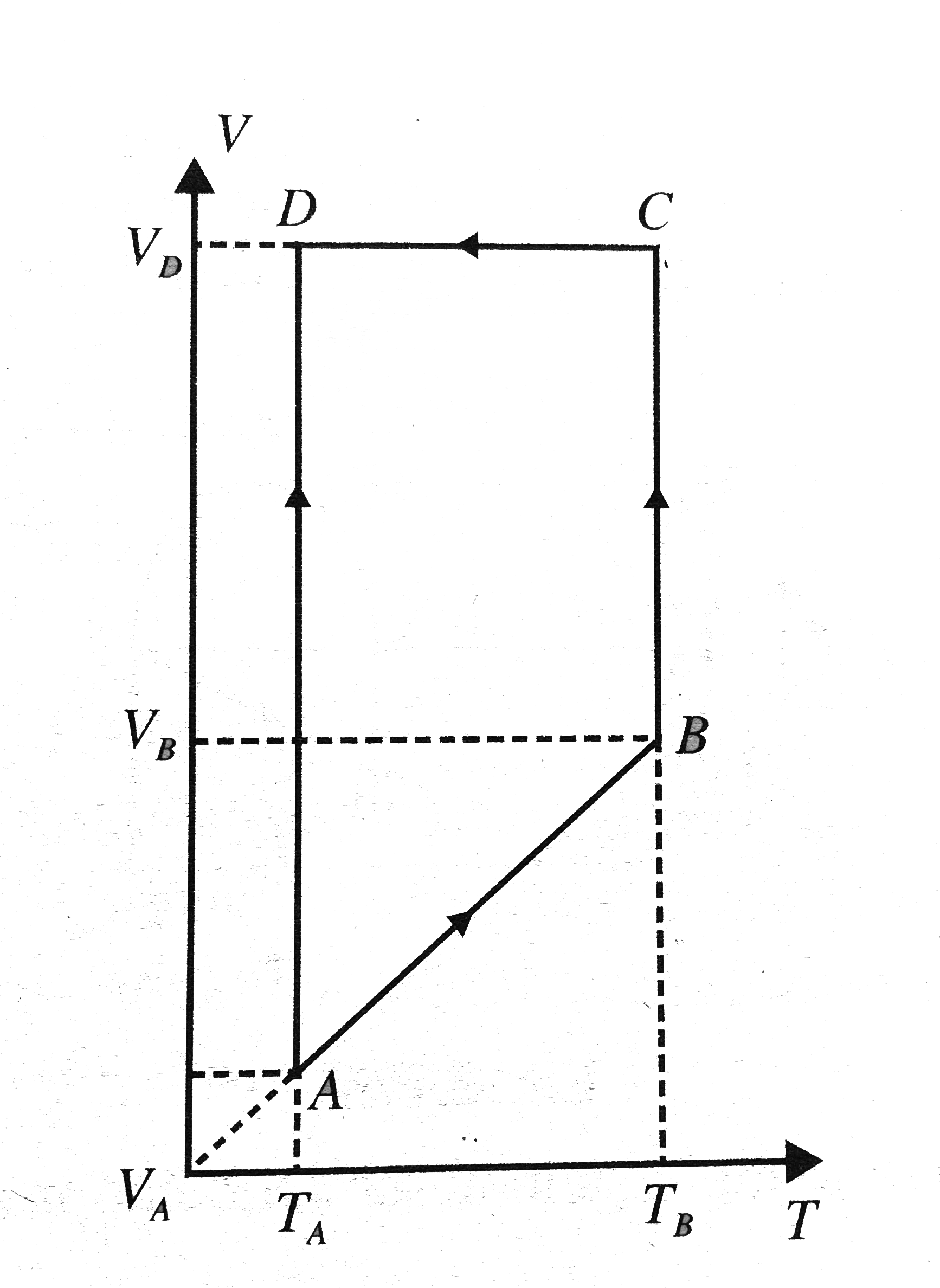
The temperature of the gas at point B
View SolutionA monatomic idea gas of 2 mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are V_(B)//V_(A)=2 and V_(D)//V_(A)=4 . If the temperature T_(A) at A is 27^(@)C , and gas constant is R . Calculate. Heat absorbed or released by the gas in process A rarr B
View SolutionA monatomic idea gas of 2mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are VB/VA=2 and VD/VA=4. If the temperature TA at A is 27∘C, and gas constant is R. Calculate.
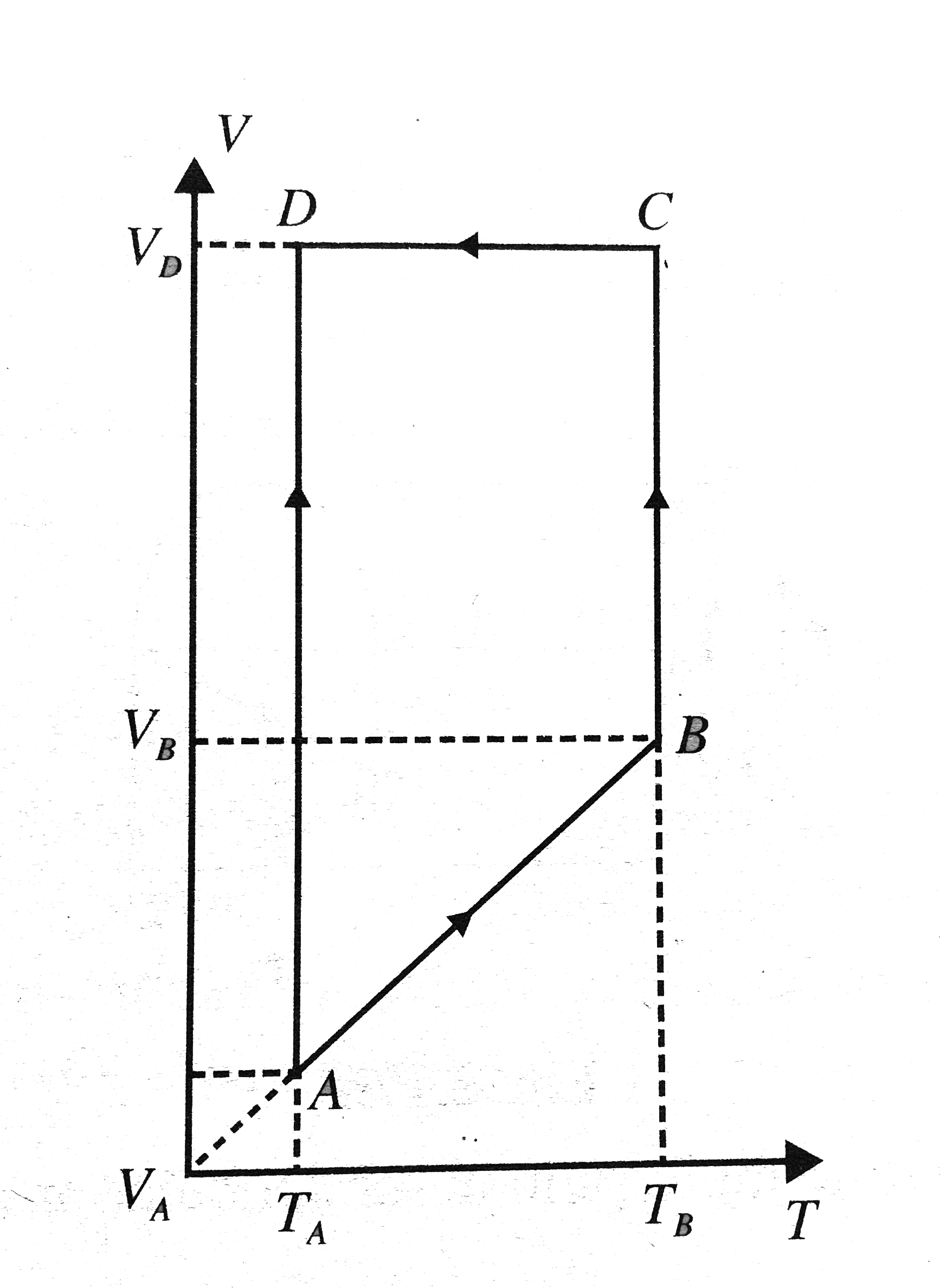 Heat absorbed or released by the gas in process B→C
Heat absorbed or released by the gas in process B→C
View SolutionA monatomic idea gas of 2 mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are V_(B)//V_(A)=2 and V_(D)//V_(A)=4 . If the temperature T_(A) at A is 27^(@)C , and gas constant is R . Calculate. Heat absorbed or released by the gas in process C rarr D .
View SolutionA monatomic idea gas of 2mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are VB/VA=2 and VD/VA=4. If the temperature TA at A is 27∘C, and gas constant is R. Calculate.
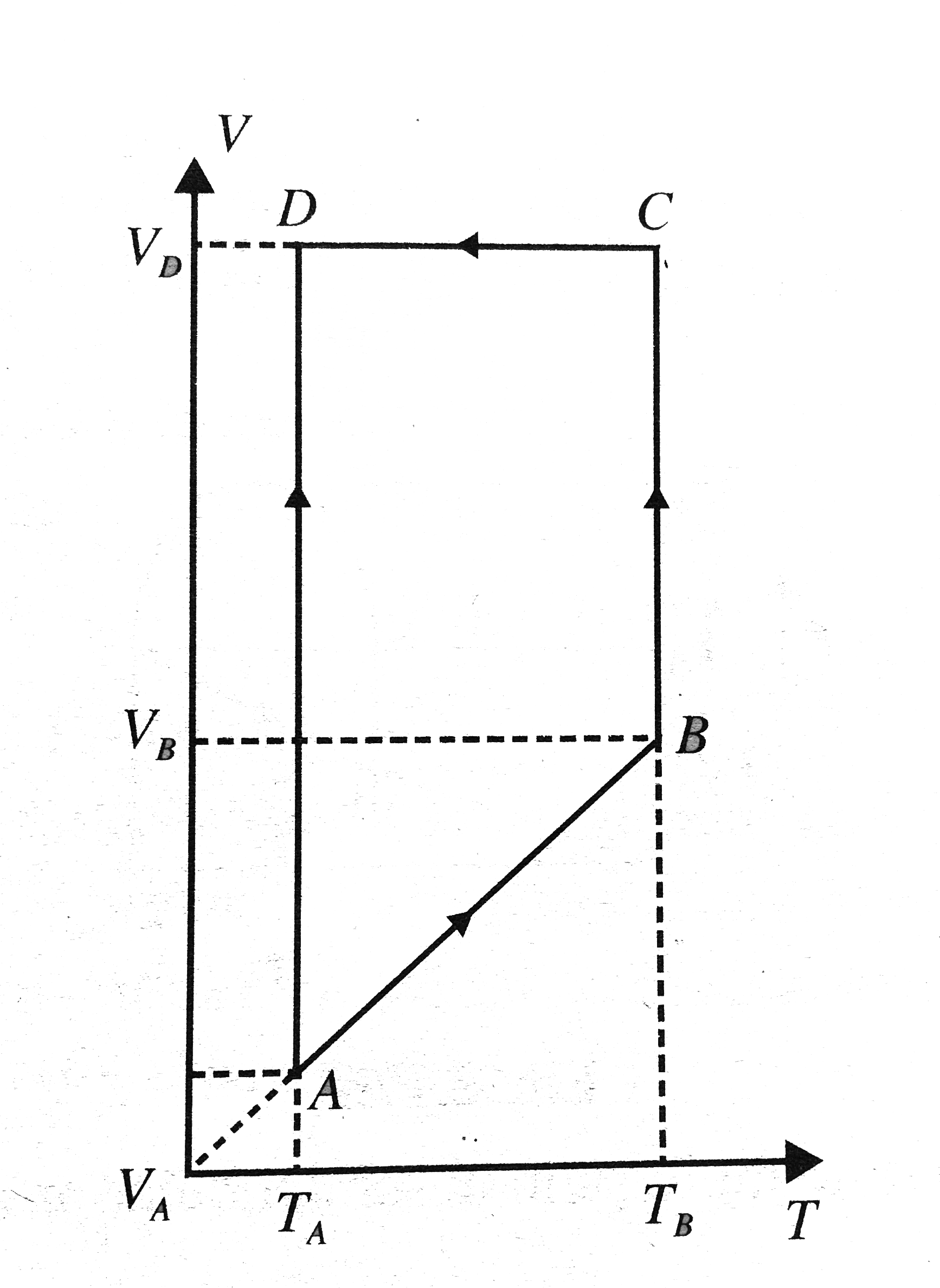
Heat absorbed or released by the gas in procedd D→A.
View SolutionA monatomic idea gas of 2 mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are V_(B)//V_(A)=2 and V_(D)//V_(A)=4 . If the temperature T_(A) at A is 27^(@)C , and gas constant is R . Calculate. The total work done by the gas during the complete cycle.
View SolutionA monoatomic ideal gas of two moles is taken through a cyclic process starting from A as shown in figure. The volume ratios are (V_(B))/(V_(A))=2 and (V_(D))/(V_(A))=4 . If the temperature T_(A) at A is 27^(@)C , calculate: (a) The temperature of the gas at point B . (b) Heat absorbed or released by the gas in each process. (c) The total work done by the gas during complete cycle.
View Solution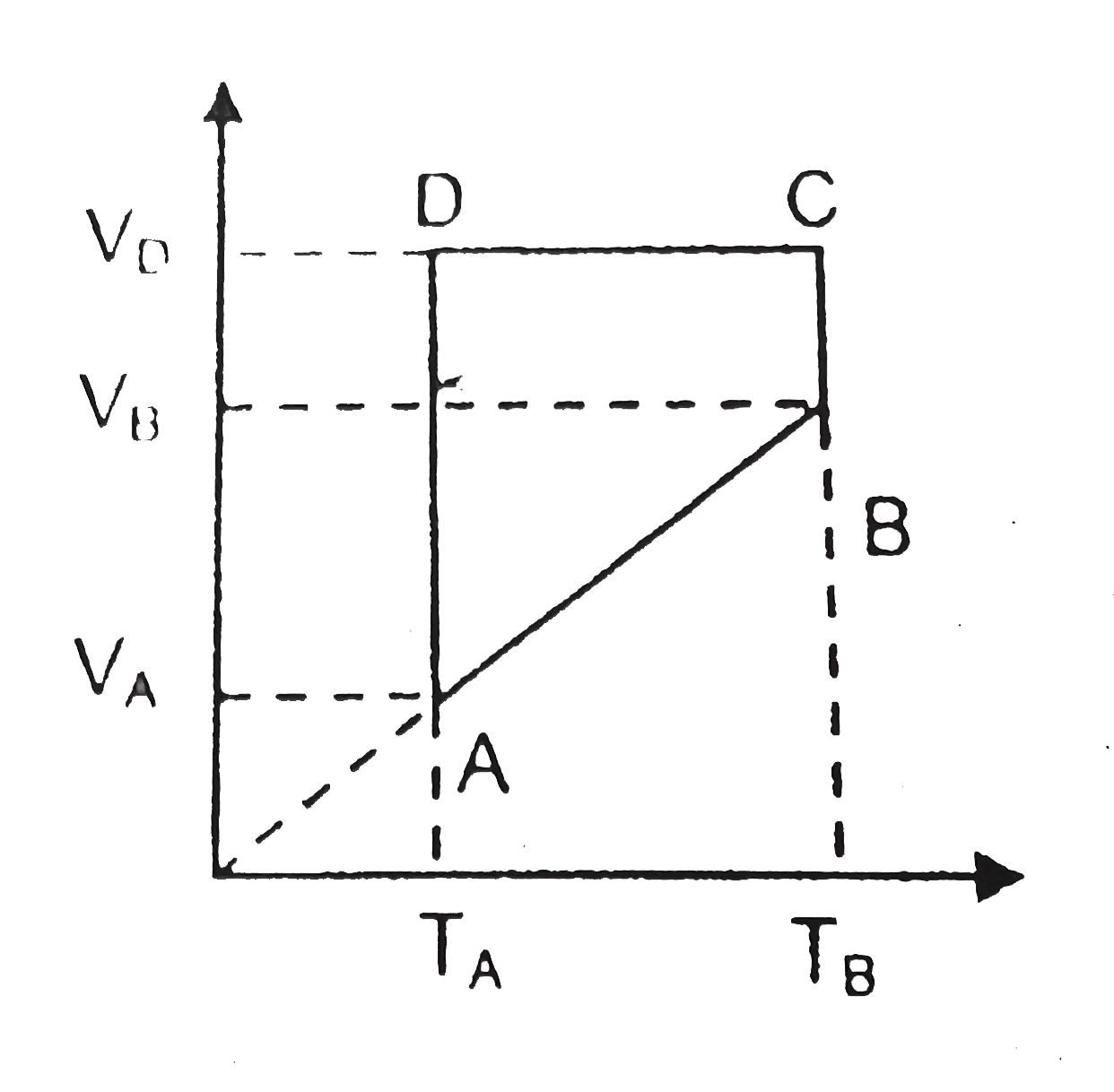
A monoatomic ideal gas of two moles is taken through a cyclic process starting from A as shown
VBVA=2 and VDVA=4
Temperature TA at A is 27∘C.
Q. Temperature at C is
View SolutionA monoatomic ideal gas of two moles is taken through a cyclic process starting from A as shown figure. The volume rations are (V_(B))/(V_(A))=2 and (V_(D))/(V_(A))=4 If the temperature fT_(A)at A is 27^(@)C . Calculate,
View Solutionदो मोल एकपरमाणुक आदर्श गैस, बिन्दु A से प्रारम्भ करके चित्रानुसार एक चक्रीय प्रक्रम में ले जायी जाती है | आयतन अनुपात (VBVA)=2 तथा (VDVA)=4 है | यदि बिन्दु A पर ताप TA=27∘C है तो गणना कीजिए
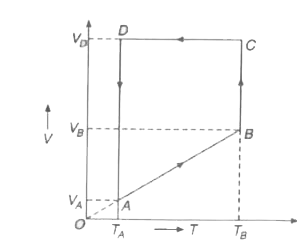
(a) बिन्दु B पर गैस का ताप
(b) प्रत्येक प्रक्रम में गैस द्वारा अवशोषित अथवा मुक्त ऊष्मा
(c ) सम्पूर्ण चक्र के दौरान दौरान गैस द्वारा किया गया कुल कार्य (अपना उत्तर गैस - नियतांक R के पदों में व्यक्त कीजिए)
View SolutionA monatomic idea gas of 2mol is taken through a cyclic process starting from A as shown in figure. The volume ratio are VB/VA=2 and VD/VA=4. If the temperature TA at A is 27∘C, and gas constant is R. Calculate the temperature of the gas at point B
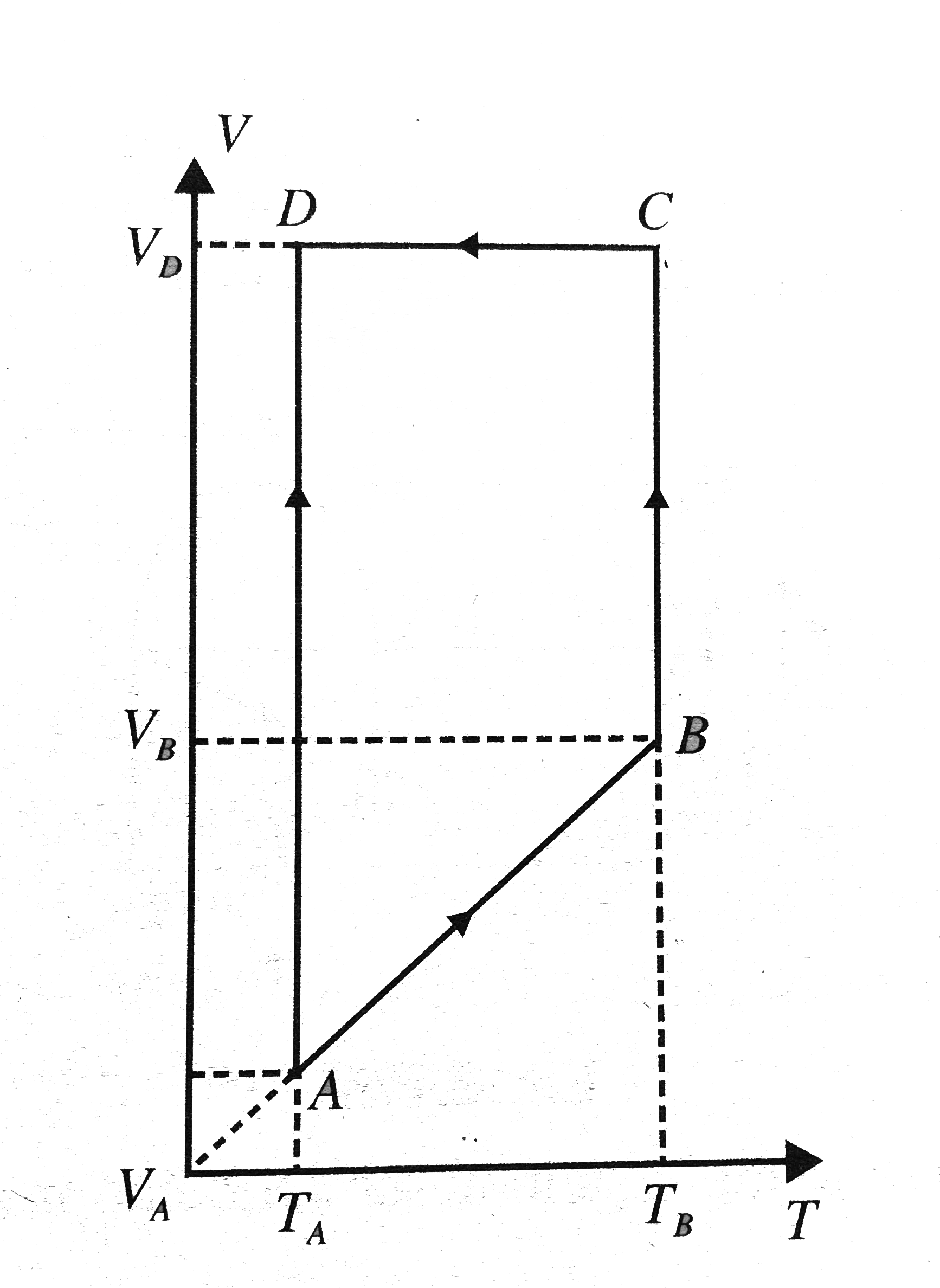
View Solution
