A moving hydrogen atom makes a head on collision with a stationary hydrogen atom. Befor collision both atoms are in in ground state and after collision they move together. The minimum value of the kinetic energy of the moving hydrogen atom, such that one of the atoms reaches one of the excitation state is
Let K be the kinetic energy of the moving hydrogen atom K' the kinetic energy of combined mass after collision.
From conservation of liner momentum .
p=p'⇒√2Km=√2K'(2m)
or K=2K'
From conservation of energy
K=K'+ΔE
Solving Eqs. (i) and (ii), we get ΔE=K2
Now, minimum value of ΔE for hydrogen atom is 10.2eV.
or ΔE≥10.2eV
∴K2≥10.2eV
∴K≥20.4eV
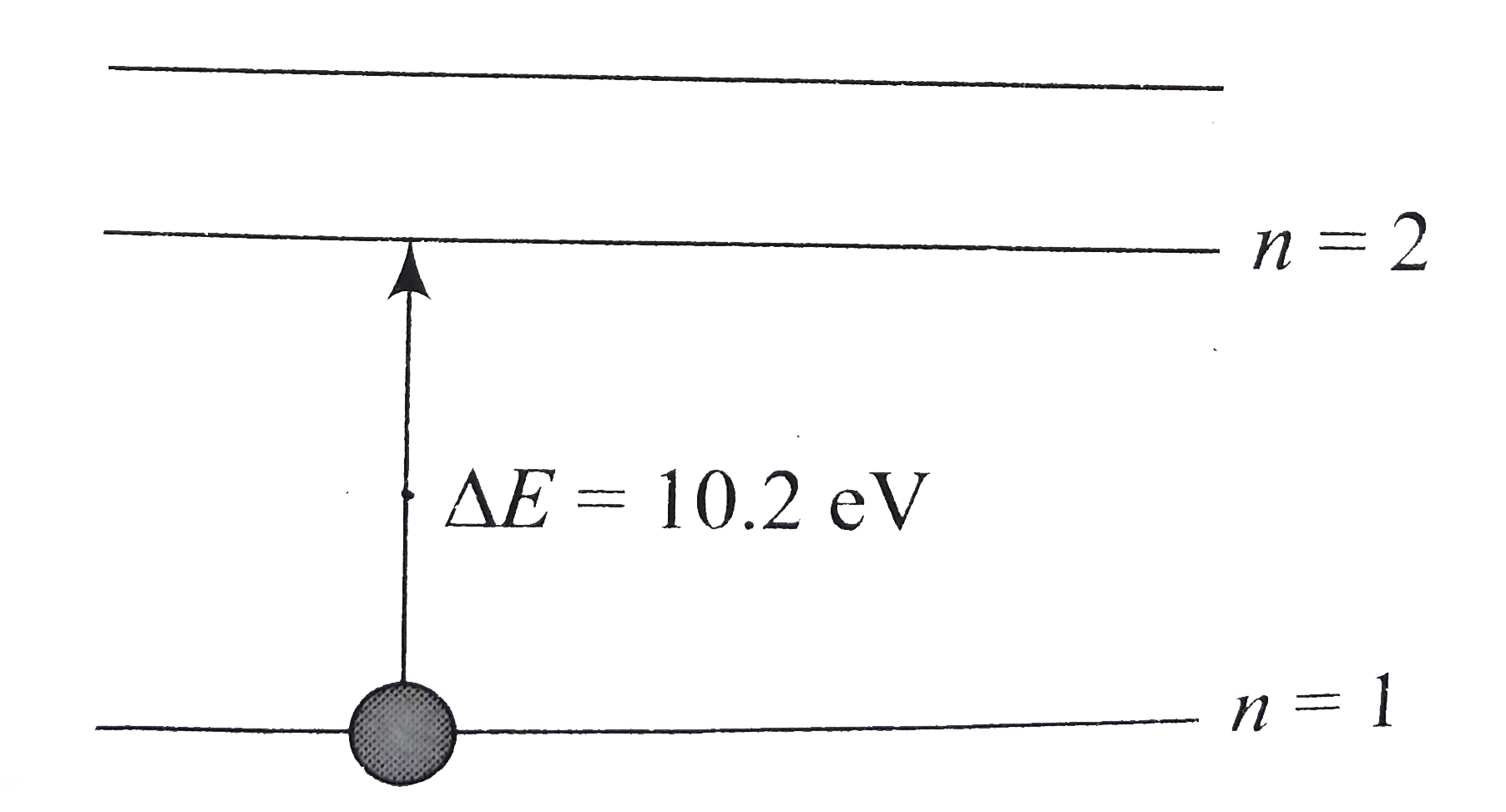
Therefore, the minimum kinetic energy of moving hydrogen is 20.4eV
Topper's Solved these Questions
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:Solved Examples
Explore 23 Videos
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:Exercise 4.1
Explore 15 Videos
- ALTERNATING CURRENTBook:CENGAGE PHYSICS ENGLISHChapter:ALTERNATING CURRENTExercise:Single Correct
Explore 10 Videos
- CAPACITOR AND CAPACITANCEBook:CENGAGE PHYSICS ENGLISHChapter:CAPACITOR AND CAPACITANCEExercise:Integer
Explore 5 Videos
Similar Questions
A moving hydrogen atom makes a head on collision with a stationary hydrogen atom. Before collision both atoms are in in ground state and after collision they move together. What is the minimum value of the kinetic energy of the moving hydrogen atom, such that one of the atoms reaches one of the excited state?
View SolutionA moving hydrogen atom makes a head on collision with a stationary hydrogen atom. Befor collision both atoms are in in ground state and after collision they move together. What is the minimum value of the kinetic energy of the moving hydrogen atom, such that one of the atoms reaches one of the excited state?
View Solution
Knowledge Check
The energy of state s1 in units of the hydrogen atom ground state energy in
A0.75B1.5C2.25D4.5
A moving hydrogen atom makes a head on collision with a stationary hydrogen atom. Before collision both atoms are in in ground state and after collision they move together. What is the minimum value of the kinetic energy of the moving hydrogen atom, such that one of the atoms reaches one of the excited state?
View SolutionA moving hydrogen atom makes a head on collision with a stationary hydrogen atom. Before collision both atoms are in in ground state and after collision they move together. What is the minimum value of the kinetic energy of the moving hydrogen atom, such that one of the atoms reaches one of the excited state?
View SolutionLet the potential energy of the hydrogen atom in the ground state be zero . Then its energy in the excited state will be
View SolutionAn electron of kinetic energy K collides elastically with a stationary hydrogen atom in the ground state. Then,
View SolutionAn electron with kinetic energy 5eV is incident on a hydrogen atom in its ground state.The collision
View SolutionAn electron with kinetic energy 5eV is incident on a hydrogen atom in its ground state.The collision
View SolutionA hydrogen atom having kinetic energy E collides with a stationary hydrogen atom. Assume all motions are taking place along the line of motion of the moving hydrogen atom. For this situation, mark out the correct statement (s).
View Solution
