A nucleus 11Na24 emits a beta particle to change into Magnesium (Mg).
Write the symbolic equation for the process.
Step by step video solution for A nucleus ""_(11)Na^(24) emits a beta particle to change into Magnesium (Mg). Write the symbolic equation for the process. by Physics experts to help you in doubts & scoring excellent marks in Class 10 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Name the type of single pulley that can act as a force multiplier. Draw a labelled diagram of the above named pulley.
View SolutionDefine .joulė..the S.I. unit of work and establish a relationship between the S.I. and C.G.S. units of work.
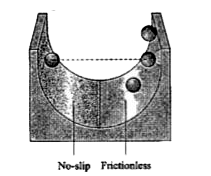
View SolutionState the efficiency of the following system as shown in the figure:
View SolutionThe nucleus .^(23)Ne deacays by beta -emission into the nucleus .^(23)Na . Write down the beta -decay equation and determine the maximum kinetic energy of the electrons emitted. Given, (m(._(11)^(23)Ne) =22.994466 am u and m (._(11)^(23)Na =22.989770 am u . Ignore the mass of antineuttino (bar(v)) .
View Solutionनिम्नलिखित नाभिकों में से समभारिकों एवं समन्यूट्रॉनिकों के जोड़े चुनकर लिखिए :
11Na23,12Mg24,11Na24,10Ne23.
View Solution(i) रेडियोऐक्टिव क्षय नियम के लिए व्यंजक N=N0e−λt को व्युत्पन्न कीजिए |
(ii) 2211Na के β+ - क्षय को व्यक्त करने की प्रक्रिया को सांकेतिक रूप में लिखिए | इस क्षय से संबंधित मूल नाभिकीय प्रक्रिया को भी लिखिए |
(iii) नाभिक 2211Na के क्षय से निर्मित नाभिक समस्थानिक है या समभारिक ?
View Solutionनिम्नलिखित अभिक्रियाएँ पूर्ण कीजिए :
2411Na→2412Mg+ ……………..
View Solution._(11) ^(23)Na and _(12)^(24)Mg are
View SolutionState the law of radioactivity and hence, show that N=N0e−λt.
View SolutionThe nucleus .^(23)Ne deacays by beta -emission into the nucleus .^(23)Na . Write down the beta -decay equation and determine the maximum kinetic energy of the electrons emitted. Given, (m(._(11)^(23)Ne) =22.994466 am u and m (._(11)^(23)Na =22.989770 am u . Ignore the mass of antineuttino (bar(v)) .
View Solution(a) Deduce the expression, N= N_(0) e^(-lambdat) for the law of radioactive decay. (b) (i) Write symbolically the process expressing the beta^(+) decay of " "_(11)^(22)Na . Also write the basic nuclear process underlying this decay. (ii) Is the nucleus formed in the decay of the nucleus " "_(11)^(22)Na , an isotope or isobar ?
View Solutionbeta - Particles does not exist inside a nucleus. But it is emitted from the nucleus! Why beta particle is emitted from the nucleus?
View SolutionA nucleus ""_(11)Na^(24) emits a beta -particle to change into magnesium (Mg). What are numbers 24 and 11 called?
View SolutionA nucleus ""_(11)Na^(24) emits a beta particle to change into Magnesium (Mg). Write the symbolic equation for the process.
View SolutionA nucleus ""_(11)Na^(24) emits a beta particle to change into Magnesium (Mg). What is the general name ""_(12)^(24)Mg with respect to ""_(11)^(24)Na ?
View SolutionWhat is the change of atomic number Z of a nucleus when it emits a β particle?
View Solution
