A process in which work perfomed by an ideal gas is proportional to the corresponding increment of its internal energy is described as a polytropic process. If we represent work done by a ppolytropic process by W and increase in internal energy as ΔU then
W∝ΔU
or W=K1ΔU ...(i)
For this process, it can be demonstrated that the relation between pressure and volume is given by the equation
PVη=K2 (constant ) .....(ii)
We know that a gas can have various values for molar specific heats. The molar specific heat 'C' for an ideal gas in polytropic process can be calculated with the help of first law of thermodynamics. In polytropic process process the variation of molar specific heat 'C' with η for a monatomic gas is plotted as in the graph shown.
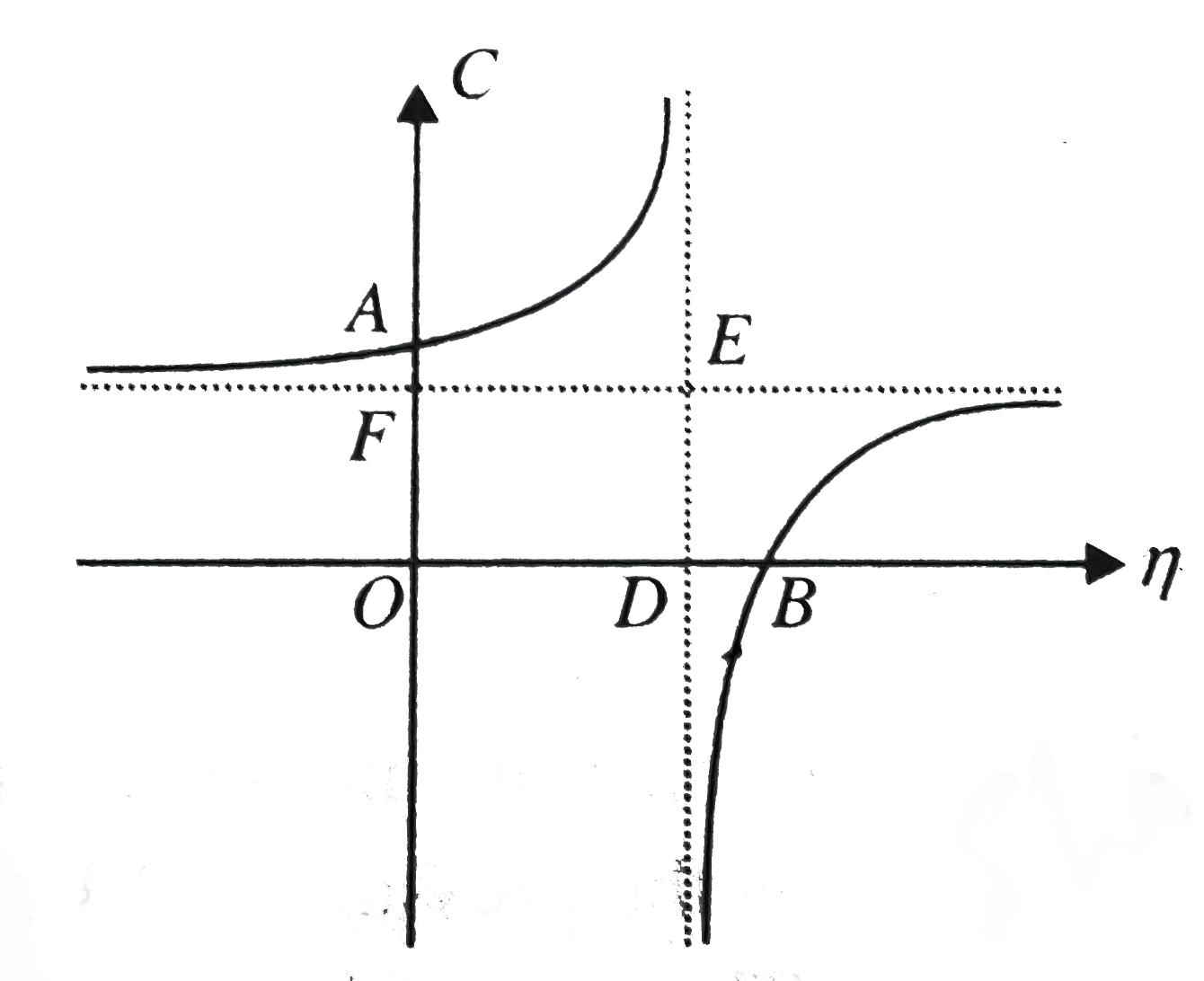
For a monoatomic gas, the values of polytropic constant η for which value specific heat is negative.
1<η<23
1<η<83
1<η<53
23<η<83
The correct Answer is:C
C→±α
is negative for 1<η<γ
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Interger
Explore 11 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Assertion-Reasoning
Explore 6 Videos
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASESExercise:Compression
Explore 2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBook:CENGAGE PHYSICS ENGLISHChapter:LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONExercise:Single correct anwer type
Explore 14 Videos
Similar Questions
8g of oxygen, 14 g of nitrogen and 22 g carbon dioxide are mixed in an encloser of volume 10 litre and temperature 27∘C. Calculate the pressure exerted by the mixture , R=8.3Jmole−1K−1, Molecular weight of oxygen , nitrogen and carbon 32 , 28 and 44 respectively.
View SolutionStatement I: A quasi -static process is so called bacause it is a sudden and large change of the system.
Statement II: An adiabatic process is not quasi- static because it is a sudden and large change of the system.
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
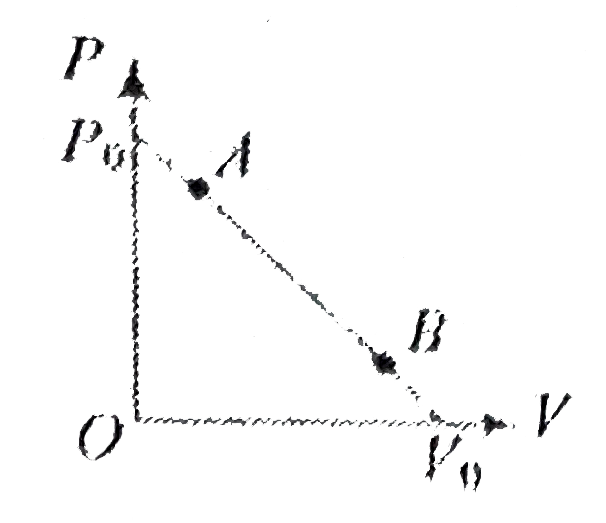
The absolute temperature T versus volume V for the given process is
View SolutionThe period of a simple pendulum whose bob is hollow metallic sphere is T. The period is T1 when the bob is filled with sand, T2 where it is filled with mercury and T3 when it is half filled with mercury Which of the following is true?
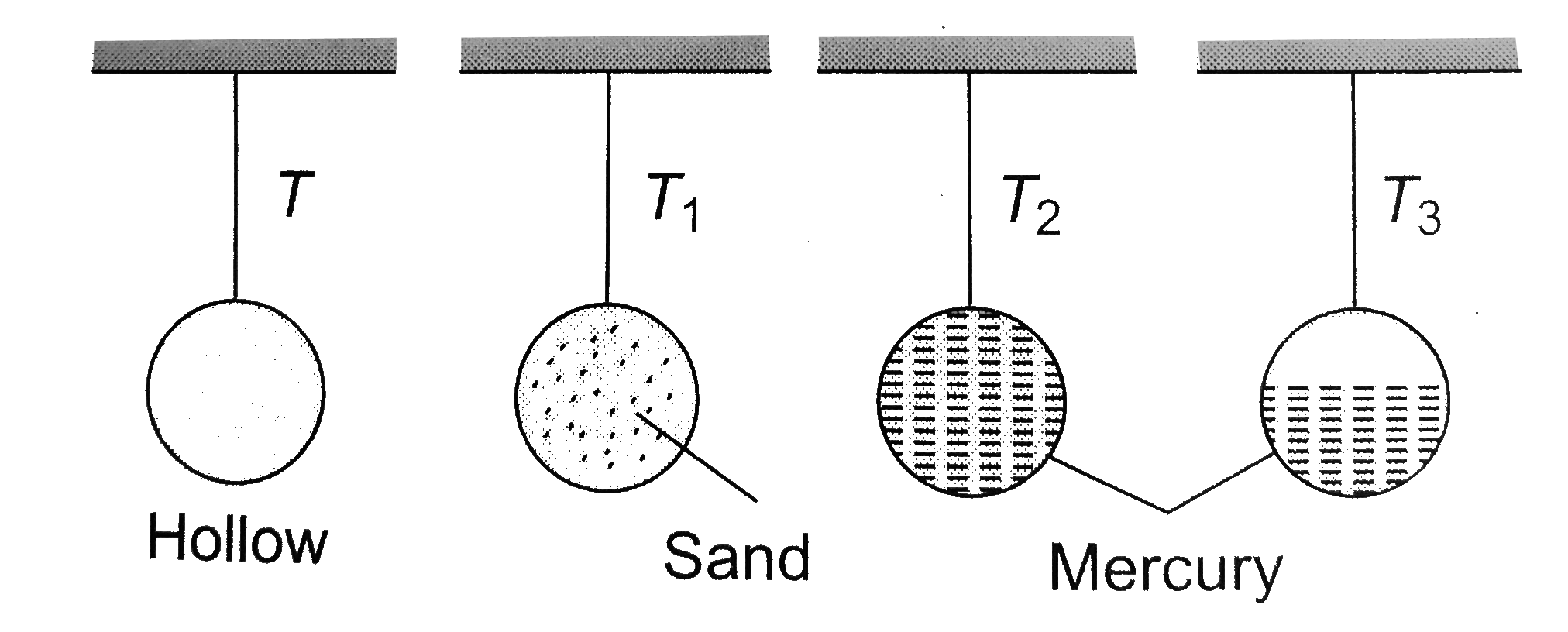
View SolutionA process in which work perfomed by an ideal gas is proportional to the corresponding increment of its internal energy is described as a polytropic process. If we represent work done by a ppolytropic process by W and increase in internal energy as Delta U then W prop Delta U or W=K_(1) DeltaU ...(i) For this process, it can be demonstrated that the relation between pressure and volume is given by the equation PV^( eta)= K_(2) (constant ) .....(ii) We know that a gas can have various values for molar specific heats. The molar specific heat 'C' for an ideal gas in polytropic process can be calculated with the help of first law of thermodynamics. In polytropic process process the variation of molar specific heat 'C' with eta for a monatomic gas is plotted as in the graph shown. In the graph shown, the y- coordinate of point A is ( for monatomic gas )
View SolutionA process in which work perfomed by an ideal gas is proportional to the corresponding increment of its internal energy is described as a polytropic process. If we represent work done by a ppolytropic process by W and increase in internal energy as Delta U then W prop Delta U or W=K_(1) DeltaU ...(i) For this process, it can be demonstrated that the relation between pressure and volume is given by the equation PV^( eta)= K_(2) (constant ) .....(ii) We know that a gas can have various values for molar specific heats. The molar specific heat 'C' for an ideal gas in polytropic process can be calculated with the help of first law of thermodynamics. In polytropic process process the variation of molar specific heat 'C' with eta for a monatomic gas is plotted as in the graph shown. In the graph shown, the y- coordinate of point A is ( for monatomic gas )
View SolutionA process in which work perfomed by an ideal gas is proportional to the corresponding increment of its internal energy is described as a polytropic process. If we represent work done by a ppolytropic process by W and increase in internal energy as Delta U then W prop Delta U or W=K_(1) DeltaU ...(i) For this process, it can be demonstrated that the relation between pressure and volume is given by the equation PV^( eta)= K_(2) (constant ) .....(ii) We know that a gas can have various values for molar specific heats. The molar specific heat 'C' for an ideal gas in polytropic process can be calculated with the help of first law of thermodynamics. In polytropic process process the variation of molar specific heat 'C' with eta for a monatomic gas is plotted as in the graph shown. In the graph shown, the y- coordinate of point A is ( for monatomic gas )
View SolutionIf DeltaU and Delta W represent the increase in internal energy and work done by the system resectively in a thermodynamical process, which of the following is true?
View SolutionAn ideal gas with the adiabatic exponent gamma undergoes a process in which its internal energy relates to the volume as u = aV^alpha . Where a and alpha are constants. Find : (a) the work performed by the gas and the amount of heat to be transferred to this gas to increase its internal energy by Delta U , (b) the molar heat capacity of the gas in this process.
View SolutionPredict the sign of q, w and Delta U for given process. Hence , find number of process for which at least one of q,w, DeltaU,DeltaH are zero.
View SolutionAn ideal gas expands from volume V _(1) to V _(2). This may be achieved by either of the three processes : Delta U be the change in internal energy of the gas, Q be the quantity of heat added to the system and W be the work done by the system on the gas. Identify which of the following statements is false for Delta U ?
View SolutionIn a process, q cal heat is absorbed by a closed system. If work done by the system is w cal, then what will be the value of the change in internal energy (ΔU) in this process?
View SolutionIf ΔU and ΔW represent the increase in internal energy and work done by the system resectively in a thermodynamical process, which of the following is true?
View SolutionThe internal energy of a system is U_(1) . In a process, work done by the system is W and heat accepted by the system is Q. At the end of the process, the internal energy of the system is
View SolutionIf ΔU and ΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true ?
View SolutionIf ΔUandΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true?
View Solution
