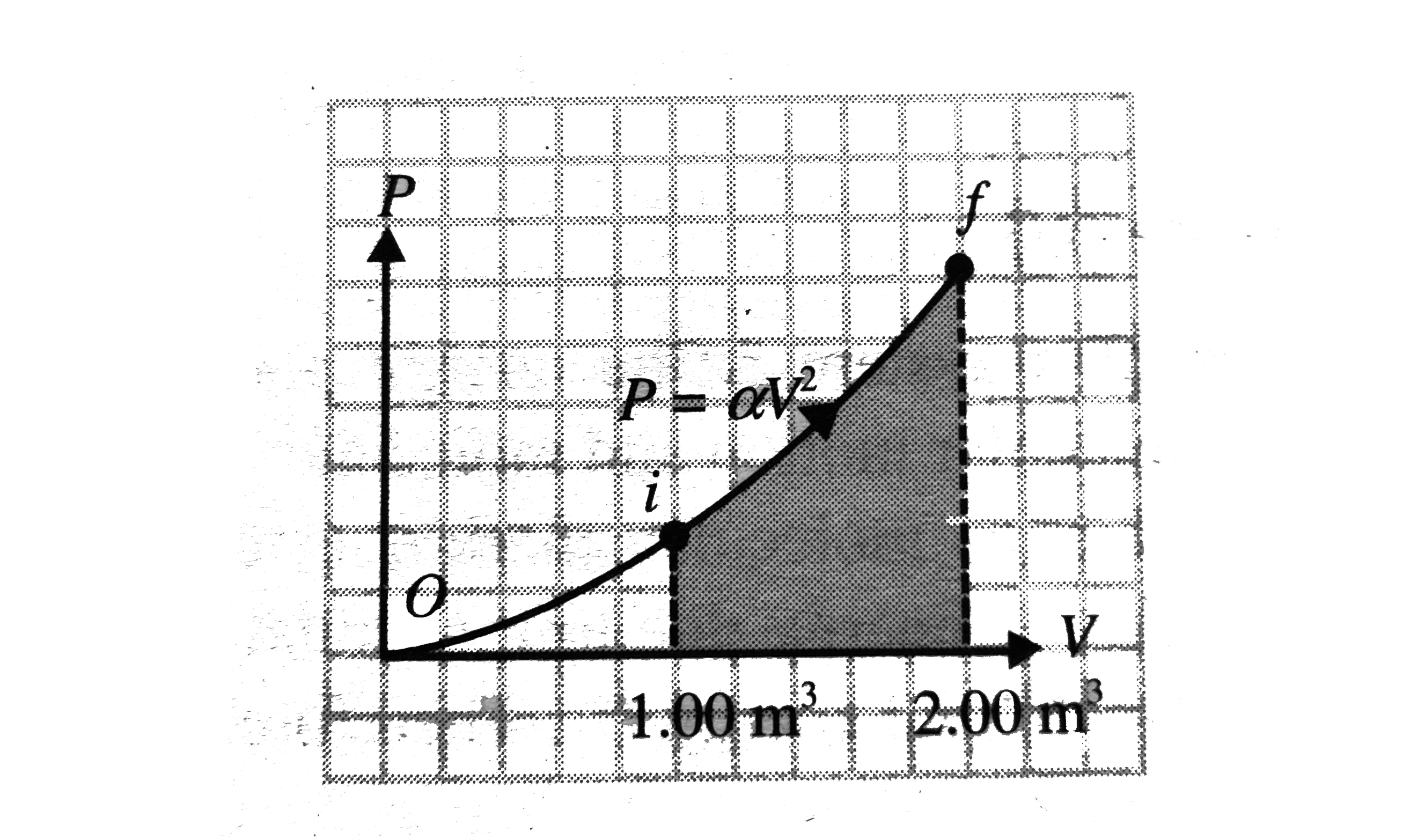
A sample of ideal gas is expanded to twice its original volume of 1.00m3 in a quasi-static process for which P=αV2, with α=5.00atm/m6, as shown in Fig. How much wor is done on the expanding gas ?
8×105J
7×105J
6×105J
3×105J
The correct Answer is:B
b. Work done, W=∫V2V1αV2dV
where α=3×105Pa/m6
⇒W=[αV33]V2=2m3V1=[3×105V33]V2V1
⇒W=105(V32−V31)
V1=1m3V2=2m3
W=105(8−1)=7×105J
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Multiple Corrects
Explore 28 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Assertion-Reasoning
Explore 6 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Subjective
Explore 22 Videos
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICS ENGLISHChapter:KINETIC THEORY OF GASESExercise:Compression
Explore 2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBook:CENGAGE PHYSICS ENGLISHChapter:LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONExercise:Single correct anwer type
Explore 14 Videos
Similar Questions
A sample of ideal gas is expanded to twice its original volume of 1.00m3 in a quasi-static process for which p=αV2, with α=5.00atm/m6, as shown in Fig. How much work is done by the expanding gas?
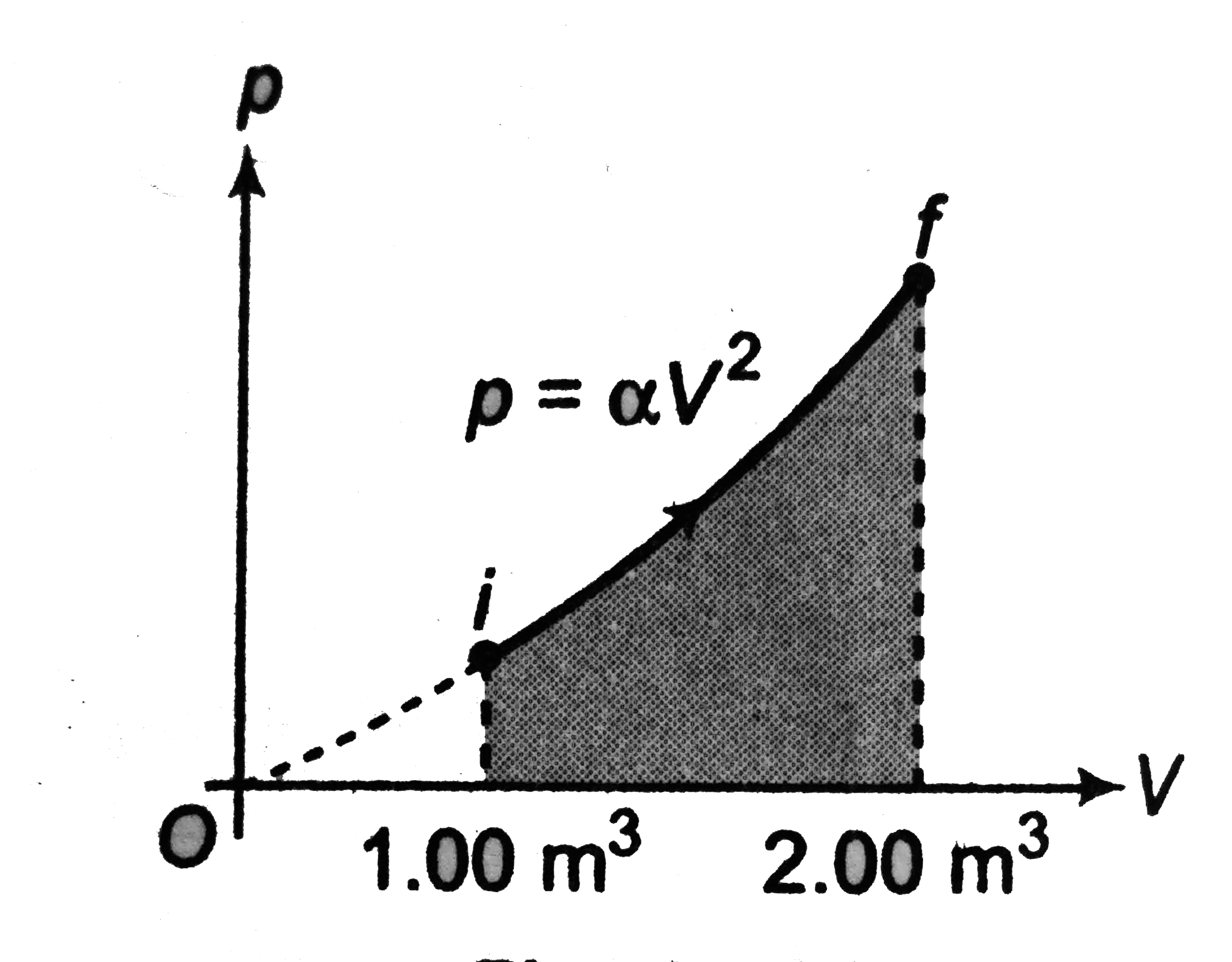
View SolutionA sample of ideal gas is expanded to twice its original volume of 1.00m3 in a quasi-static process for which p=αV2, with α=5.00atm/m6, as shown in Fig. How much work is done by the expanding gas?

View SolutionA sample of an ideal gas is expanded to twice its original volume of 1m3 in a reversible process for which P=αV2, where α=5atmm−6. If CV,m=20Jmol−1K−1, determine moalr change in entropy (ΔSm) for the process.
View SolutionAn ideal gas is taken through a quasi-static process described by P=αV2, with α=5.00atm/m6. The gas is expanded to twice its original volume of 1.00m3. How much work is done by the gas in expanding gas in this process?
View SolutionA sample of an ideal gas is expanded from 1dm3 to 3 in a reversible process for which P=KV3, with K=1/5(atm/dm3), what is work done by gas (Latm)
View SolutionA sample of an ideal gas is expanded 1m3 to 3m3 in a reversible process for which P=KV2 , with K=6bar/m6. What is work done by the gas (in kJ) ?
View SolutionOne mole of an ideal gas is expanded from a volume of 3L to 5L under a constant pressure of 1 atm. Calculate the work done by the gas.
View Solution5 mole of an ideal gas of volume is expanded against vaccum to make its volume 2 times, then work done by the gas is:
View Solution0.5 litre of an ideal gas at a pressure of 20 atm expands to a final volume of 0.5m3. The process obeys the equation PV = a constant, (i) Find the value of this constant (ii) What is the final pressure ? And (iii) Find, the work done by the gas,
View Solutionone mole an ideal gas having volume 2 litre and at a pressure of 10 atm expands isothermally at 25 °C into a vacuum until its total volume is 10 litres. How much heat is absorbed and how much work is done in the expansion ?
View Solution
