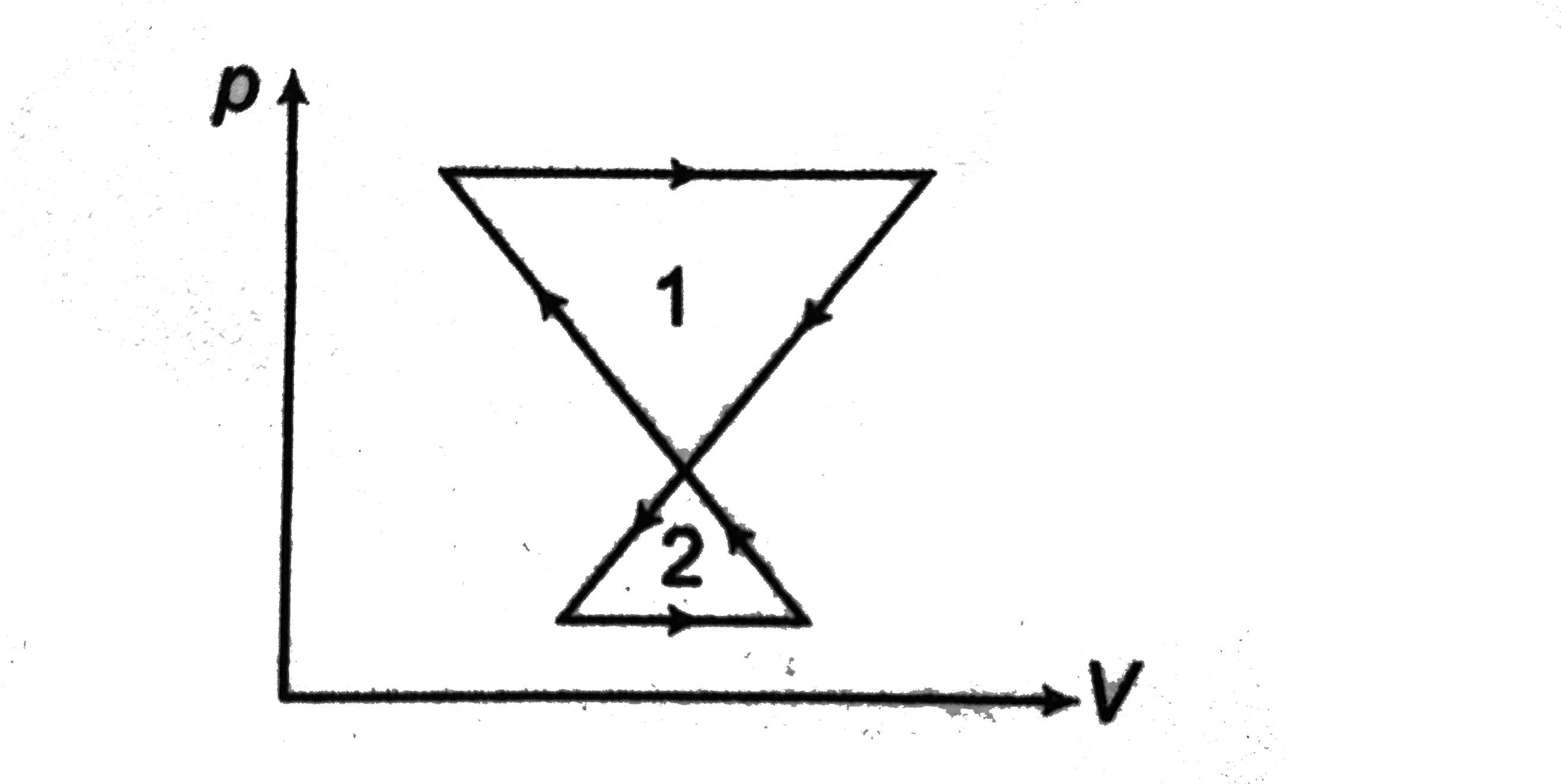
A thermodynamic system undergoes a cyclic process as shown in figure.
(a) over one complete cycle, does the system do positive or negative work.
(b) over one complete cycle, does heat flow into or out of the system.
(c) In each of the loops 1 and 2, does heat flow into or out of the system.
The correct Answer is:A, B, C
(a) W1=+ve, as cycle is clockwise
W2=-ve as cycle is anti-clockwise
Since, |W1|>|W2|
∴ Net work done by the system is positive.
(b) In cyclic process, Qnet=Wnet
Since, Wnet is positive. So Qnet is also positive. So, heat flows into the system.
(c) W1=Q1=+ve, so into the system.
W2=Q2=−ve, so out of the system.
टॉपर्स ने हल किए ये सवाल
- LAWS OF THERMODYNAMICSBook:DC PANDEY ENGLISHChapter:LAWS OF THERMODYNAMICSExercise:Exercise 21.3
Explore 7 Videos
- LAWS OF THERMODYNAMICSBook:DC PANDEY ENGLISHChapter:LAWS OF THERMODYNAMICSExercise:Exercise 21.4
Explore 8 Videos
- LAWS OF THERMODYNAMICSBook:DC PANDEY ENGLISHChapter:LAWS OF THERMODYNAMICSExercise:Exercise 21.1
Explore 1 Video
- LAWS OF MOTIONBook:DC PANDEY ENGLISHChapter:LAWS OF MOTIONExercise:Medical entrances gallery
Explore 39 Videos
- MAGNETIC EFFECT OF CURRENT AND MAGNETISMBook:DC PANDEY ENGLISHChapter:MAGNETIC EFFECT OF CURRENT AND MAGNETISMExercise:Integer type Questions
Explore 10 Videos
समान प्रश्न
A thermodynamic system undergoes cyclic process ABCDA as shown in figure. The work done by the system is
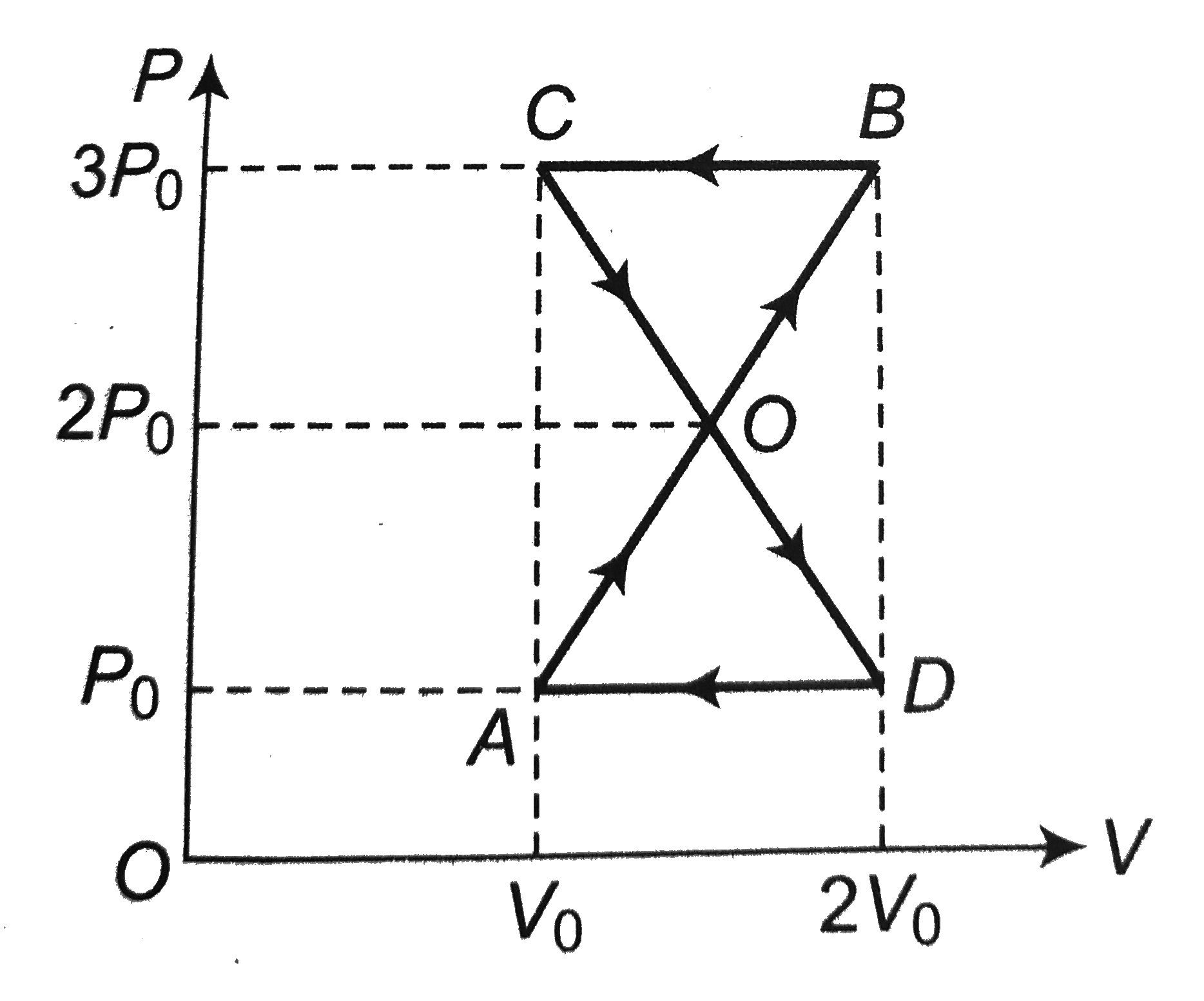
View SolutionA thermodynamic system undergoes a cyclic process as shown in figure. The cycle consists of two closed loops, loop I and loop II. (a) Over one complete cycle, does the system do positive or negative work? (b) In each of loops I and II, is the net work done by the system positive or negative? (c) Over one complete cycle, does heat flow into or out of the system? (d) In each of loops I and II, does heat flow into or out of the system?

View Solution
ज्ञान जांच
A thermodynamical system undergoes cyclic process ABCDA as shown in figure work done by the system is
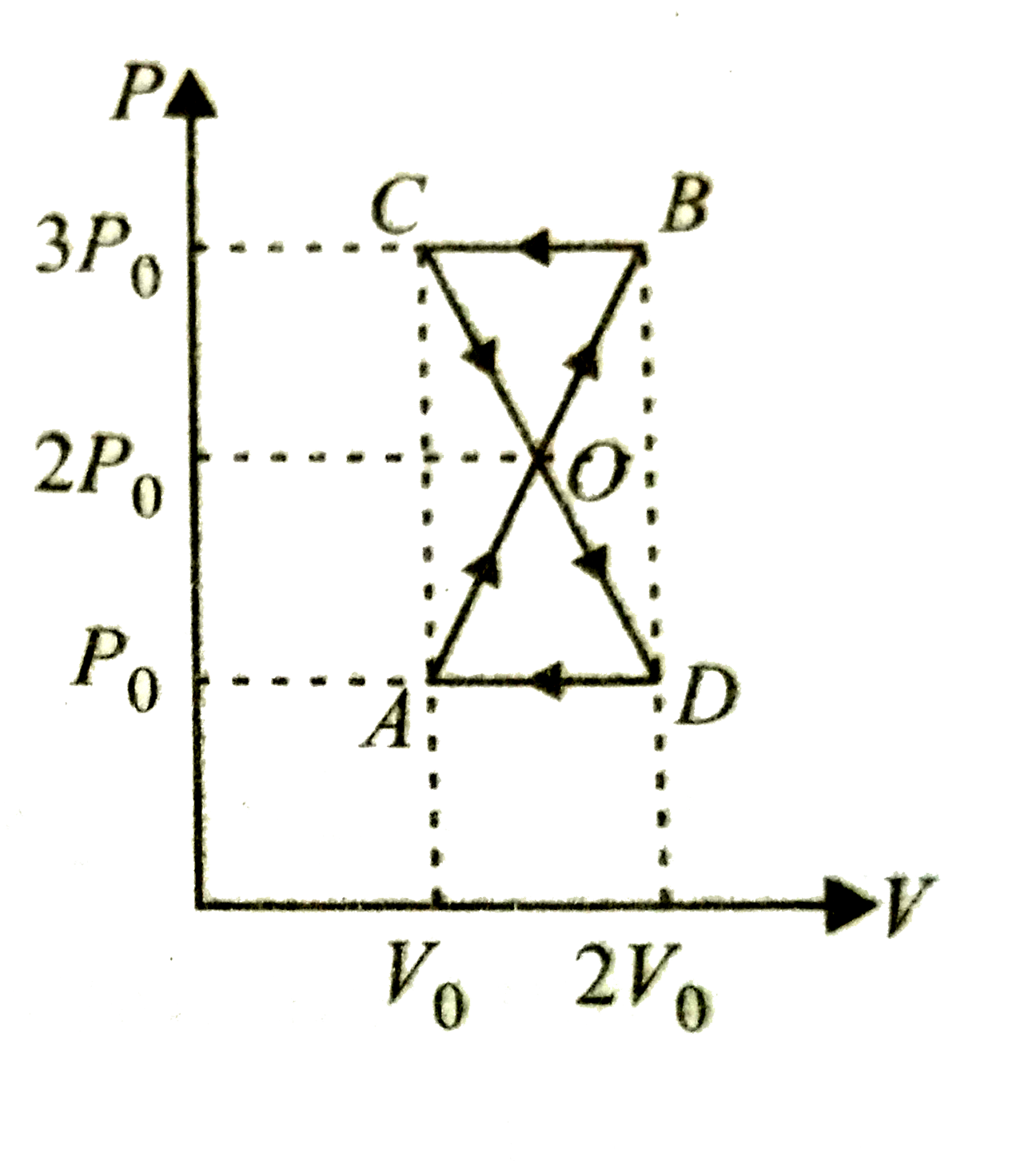 AZeroB2P0V0CP0V0D32P0V0
AZeroB2P0V0CP0V0D32P0V0
A thermodynamics system is taken trough the cycle ABCD as shown in figure. Heat rejected by the gas during the cycle is:
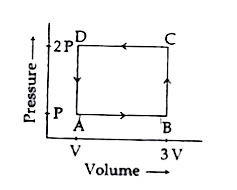
View SolutionA thermodynamic system is taken through the cycle ABCD as shown in the figure. Heat rejected by the gas during the cycle is
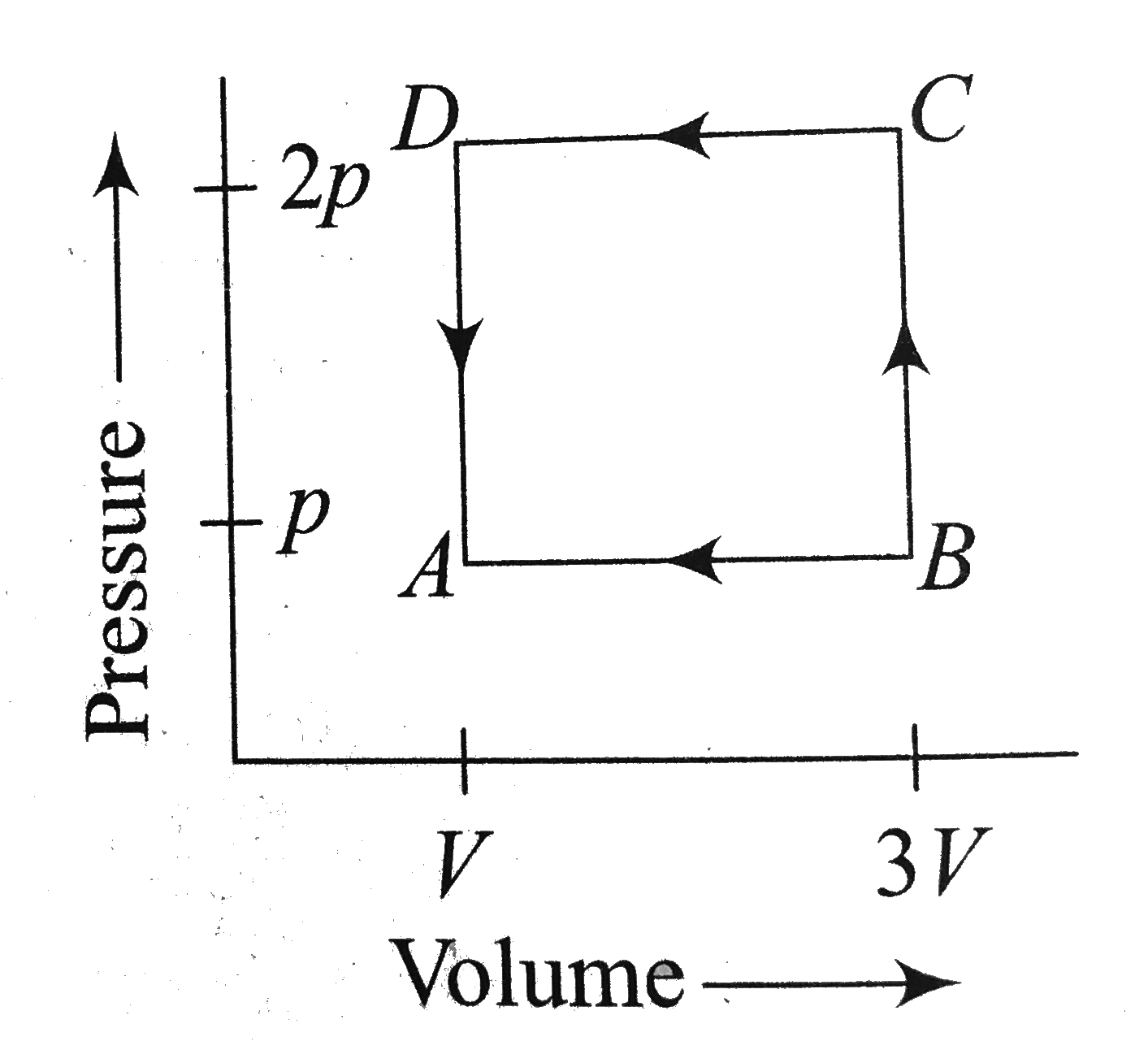
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process as shown in figure . Temperature at point 1 =300K and process 2−3 is isothermal .
_S01_113_O01.png) .
.
Net work done by gas in complete cycle is
View Solution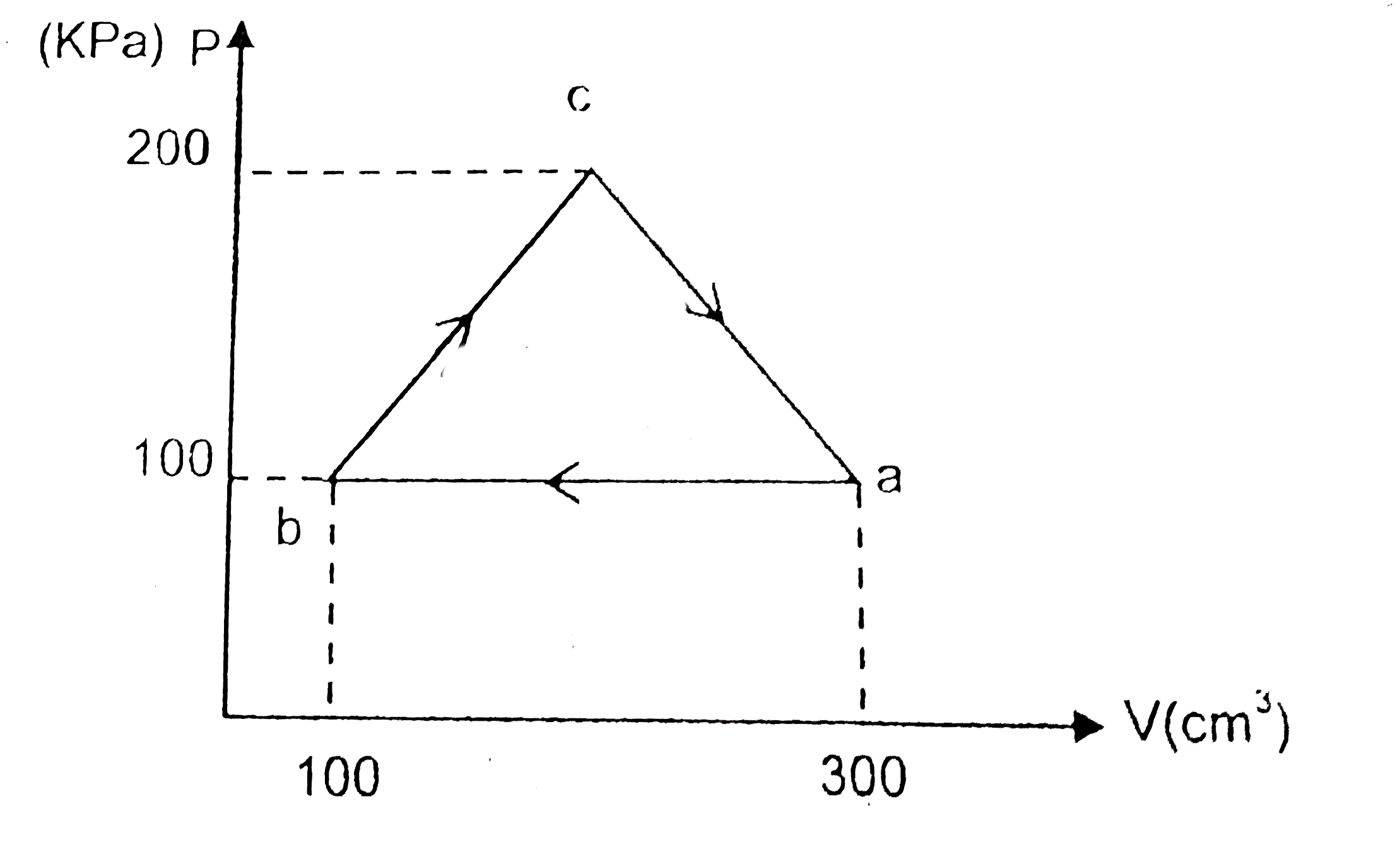
A thermodynamics system is taken through the cyclic process abca as shown in the figure. If the heat absorbed by the gas during the cycle is 5x joule then find the value of x.
View SolutionWhat is the change in the internal energy of a system over one complete cycle of a cyclic process?
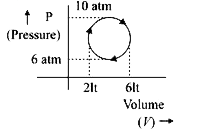
View SolutionAn ideal gas undergoes a cyclic process whose indicator diagram is as shown in the figure. Find the work done by the gas in 1 complete cycle.
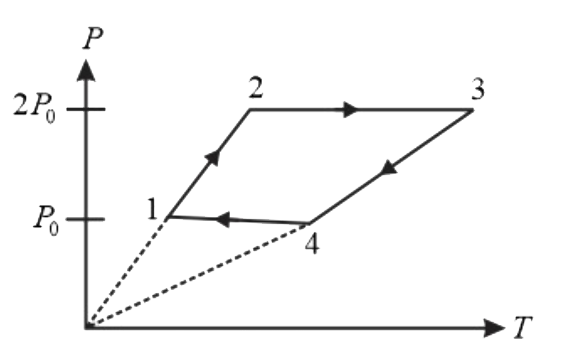
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process, as shown in the figure. If the temperature of the gas at state 1 is 300 K and at state 4 is 500 K, then heat exchanged during process 2→3, is
View Solution
