All substance expand on heating, but water shows an exception. This occur in case if temperature is from zero to what degree celsius?
The correct Answer is:4
Water on heating from 0∘C to 4∘C do not expand. So higher temperature limit of for this is 4∘C.
Topper's Solved these Questions
Similar Questions
The rate of cooling of a substance in moving air is proportional to the difference of temperatures of the substance and the air. A substance cools from 36 degree celsius to 34 degree celsius in 15 minutes. Find when the substance will have the temperature 32 degree celsius , it being known that the constant temperature of air is 30 degree celsius.
View SolutionThe given figures show the change in temperature of ice when it is heated.
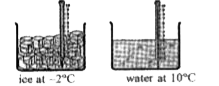
What is the change in temperature as ice converts to water?
View Solution
Knowledge Check
Which of the following are true statements?
I. The heat capacity of a substance is the amount of heat that substance can hold per unit of temperature.
II. The specic heat for a single substance is the same for all phases of that substance.
III. When heat is added to a fluid, its temperature will change less if it is allowed to expand.AOnlyBOnly IIICI and IIIDAll of theseA substance is in the solid from at 0∘C. The amount of heat added to this substance and its temperature is plotted in the graph . The specific heat capacity of the solid substance is 0.5cal/g∘C.
_E01_235_Q01.png)
Specific heat capacity in the liquid state is -A527cal/g∘CB527cal/gKC1027cal/g∘CDCan't sayA substance is in the solid from at 0∘C. The amount of heat added to this substance and its temperature is plotted in the graph . The specific heat capacity of the solid substance is 0.5cal/g∘C.
_E01_234_Q01.png)
Latest heat capacity in melting process is -A3503cal/gB1753cal/gC4003cal/gDCan't say
What happens when ferromagnetic substance is heated to high temperature?
View SolutionOn heating a ferromagnetic substance above curie temperature
View SolutionA substance is in the solid from at 0∘C. The amount of heat added to this substance and its temperature is plotted in the graph . The specific heat capacity of the solid substance is 0.5cal//g∘C.
_E01_233_Q01.png)
The mass of the substance is -
View SolutionA given substance of mass 'm' is in solid state at certain temperature. Arrange the following steps in proper sequence to calculate the total heat energy required to just convert the substance completely into gaseous state.
(A) Note down the specific latent heat of vapor ization of the substance and calculate the heat supplied to convert from liquid to gaseous state using the formula.
(B) Note down the melting point of the substance and calculate the heat supplied to increase the temperature of the solid to its melting point.
(C ) Note down the boiling point of the substance and calculate the heat supplied to increase the temperature of the substance from melting point to its boiling point.
(D) Add all the heat energies, it gives the resultant heat supplied to the solid to just convert it into gaseous state.
(E) Note down the specific latent heat of fusion of the substance and calculate the heat supplied to convert the substance from solid state to liquid state.
View SolutionA ginven substance of mass 'm' is in solid state at certain temperature. Arrange the following steps in proper sequence to calcualte the total heat energy required to just convert the substance completely into gaseous state.
(a). Note down the specific latent heat of vaporization of the substance and calculate the heat supplied to convert from liquid to gaseous state using the formula.
(b) Note down the melting point of the substance and calculate the heat supplied to increase the temperature of the solid to its melting point.
(c) Note down the boiling point of the substance and calculate the heat supplied to increase the temperature of the substance from melting point to its boiling point.
(d). Add all the heat energies, it gives the resultant hea supplied to the solid to just convert it into gaseous state.
(e) Note down the specific latent heat of fusion of the substance and calculate the heat supplied to convert the substance from solid state to liquid state.
View Solution
