An ideal gas has temperature T1 at the initial state i shown in the P- V [ diagram. The gas has a higher temperature T2 at the final states a and b, which it can reach the paths shown. The change in entropy is:
greatest in a
greatest in b
same in a and b
nothing can be said
The correct Answer is:B
Qd=ΔU+0=ΔU and Qb=ΔU+PΔV
As Qb>Qa,∴ Change in entropy is greater in case (b).
Similar Questions
A sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is TA =200 K. In states B and C the temperature of the gas is the same.
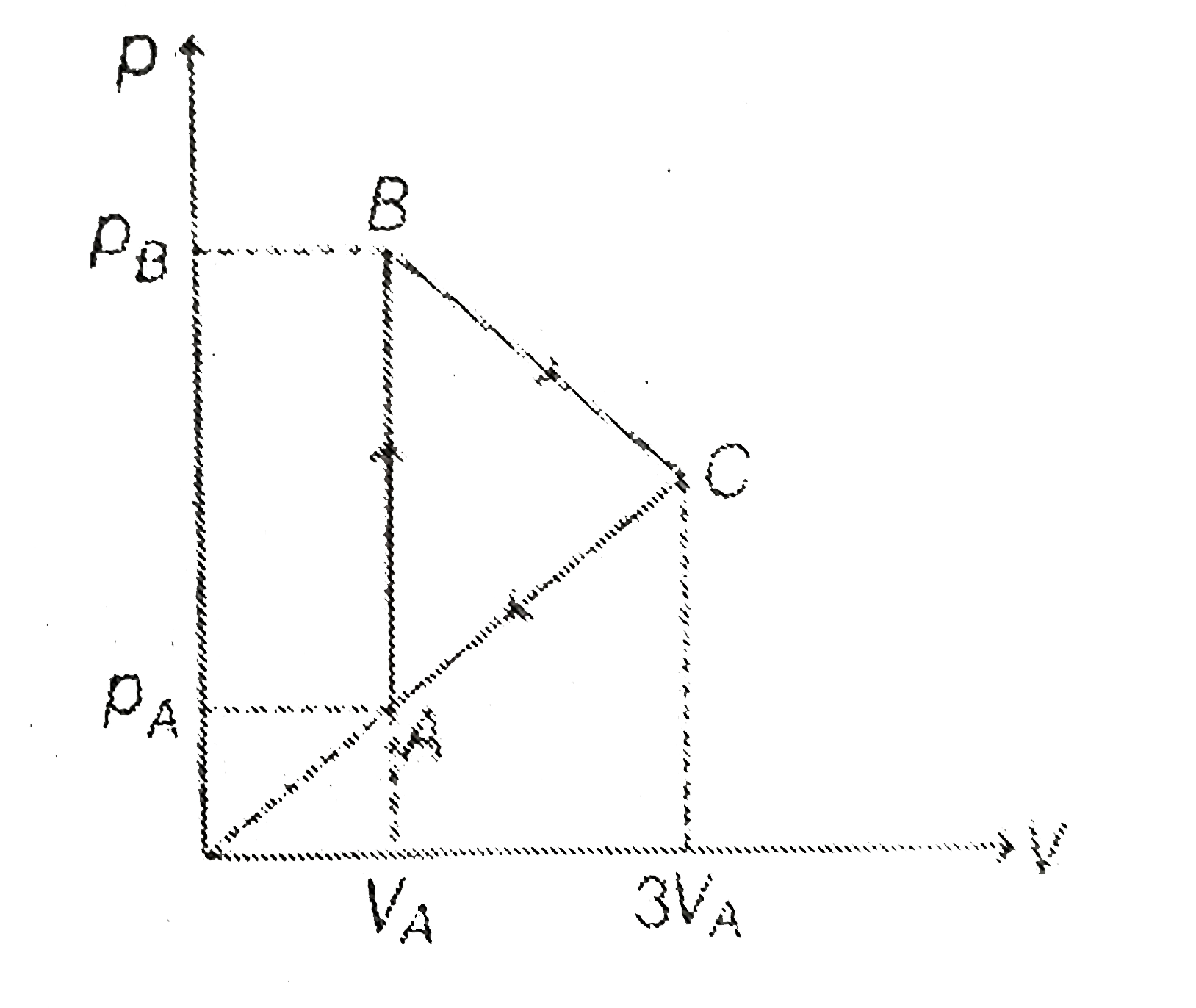
Net work done by the gas in the process is
View SolutionA sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is TA =200 K. In states B and C the temperature of the gas is the same.
Which of the following graphs best reprsent the cyclic process in T-V diagram
View Solution
Knowledge Check
An ideal gas undergoes change in its state from the initial state I to the final state F via two possible paths as shown. Then
 Athere is no change in internal energy along path 1Bheat is not absorbed by the gas in both pathsCthe temperature of the gas first increases and then decreases for path 2Dwork done by the gas is larger in path 1.
Athere is no change in internal energy along path 1Bheat is not absorbed by the gas in both pathsCthe temperature of the gas first increases and then decreases for path 2Dwork done by the gas is larger in path 1.An ideal gas is taken from state A to state B following three different paths as shown in P-V diagram. Which one of the following is true ?
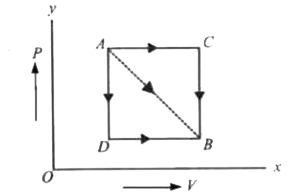 Awork done is maximum along ABBwork done is minimum along ABCwork done along ACB = work done along ADBDwork done along ADB is minimum.
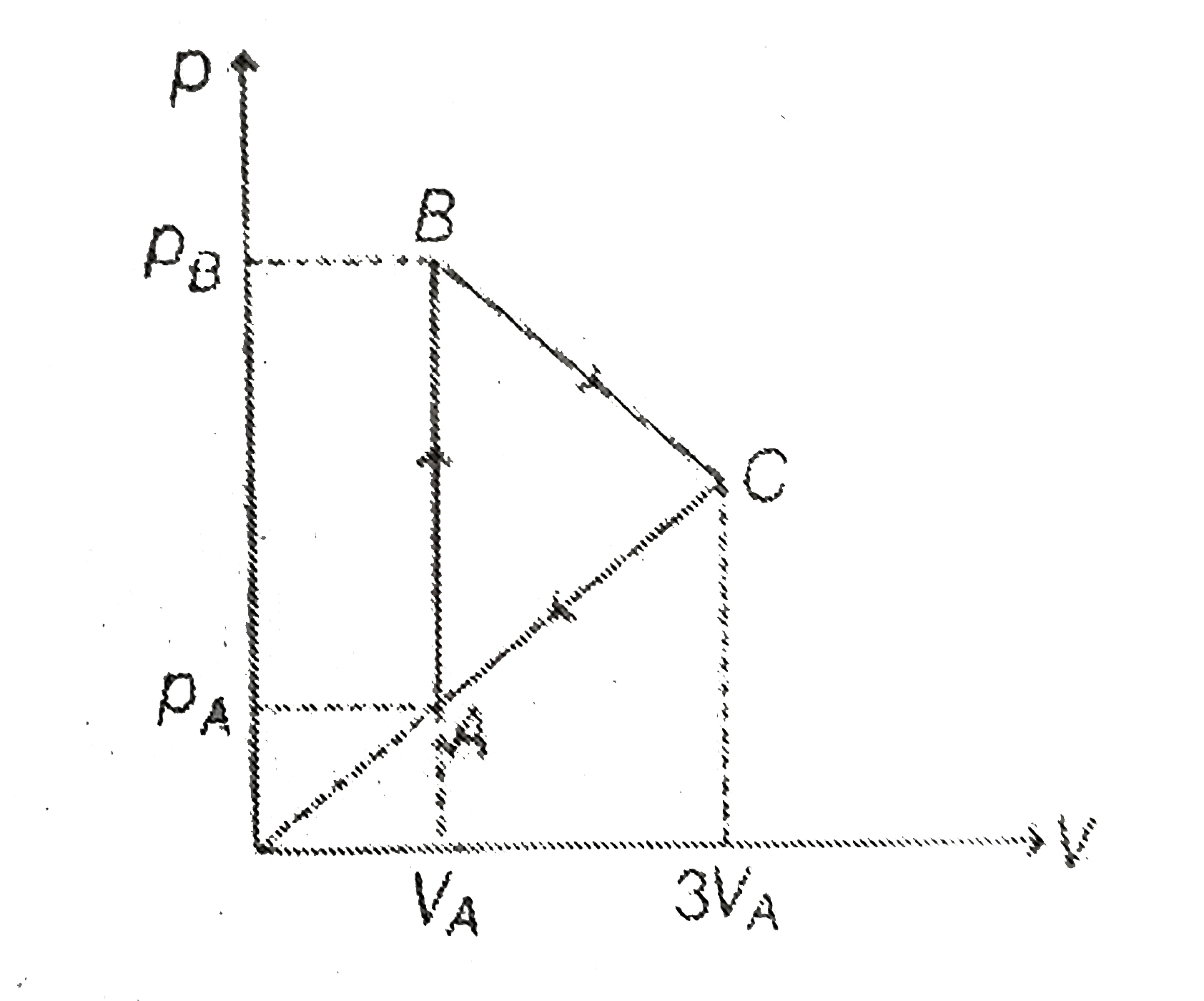
Awork done is maximum along ABBwork done is minimum along ABCwork done along ACB = work done along ADBDwork done along ADB is minimum.A sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is TA =200 K. In states B and C the temperature of the gas is the same.

Net work done by the gas in the process isA2pAVAB4pAVAC6pAVAD8pAVA
An ideal gas undergoes an expansion from a state with temperature T1 and volume V1 through three different polytropic processes A,B and C as shown in the P−V diagram. If |ΔEA|,|ΔEB| and |ΔEC| be the magnitude of changes in internal energy along the three paths respectively, then :

View SolutionA sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is TA =200 K. In states B and C the temperature of the gas is the same.

Which of the following graphs best reprsent the cyclic process in T-V diagram
View SolutionAn insulated container of gas has two chambers separated by an insulating partition. One of the chambers has volume V1 and contains ideal gas at pressure P1 and temperature T1. The other chambers has volume V2 and contains ideal gas at pressure P2 and temperature T2. If the partion is removed without doing any work on the gas, the final equilibrium temperature of the gas in the container will be
View SolutionA sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is TA =200 K. In states B and C the temperature of the gas is the same.

What is the greastes temperature of the gas during the cyclic process ?
View SolutionAn ideal gas undergoes an expansion from a state with temperature T1 and volume V1 through three different polytropic processes A,B and C as shown in the P−V diagram. If |ΔEA|,|ΔEB| and |ΔEC| be the magnitude of changes in internal energy along the three paths respectively, then :

View Solution

