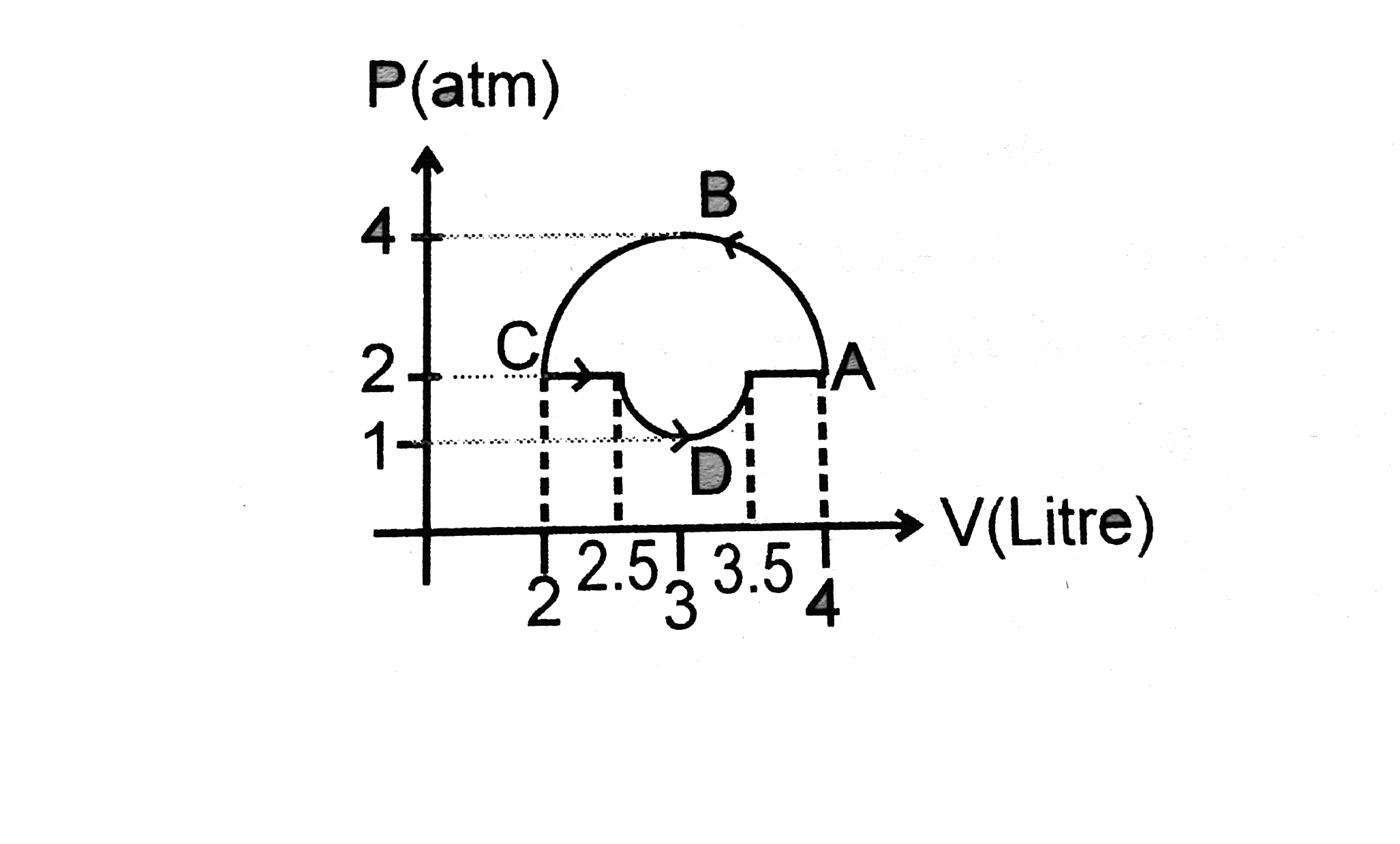
An ideal gas is taken in a cyclic process as shown in the figure. Calculate
(a) work done by the gas. (b) work done on the gas (c ) Net work done in the process Solution in Tamil
Work done by the gas (along AB)
W=P×ΔV=600×3=1800J,W=1.8kJ
(b) Work is done on the gas (along BC)
W=−P×ΔV=−400×3=−1200J
W=−1.2kJ
(c ) Net work done in the process = Area under the curve AB
=Rectangle area +triangle area
=(l×b)+(12×b×h)=(400×3)+(12×3×200)
=1200+300=1500J, W=1.5kJ

Similar Questions
एक आदर्श गैस के चित्रानुसार दिये गये चक्रीय प्रक्रम में गैस द्वारा किया गया कार्य है :
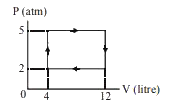
View SolutionOne mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in figure. Calculate
The work done by the gas
View SolutionA sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is T_(A) =200 K. In states B and C the temperature of the gas is the same. Net work done by the gas in the process is
View SolutionFind work done by the gas in the process shown in figure.
View SolutionFind the work done by gas going through a cyclic process shown in figure?
View SolutionFind work done by the gas in the process shown in figure.
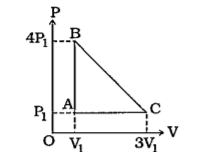
View SolutionAn ideal gas is taken around the cycle ABCA as: . The work done in the cyclic process is :
View Solutionઆકૃતિમાં દર્શાવેલ આદર્શ વાયુની ચક્રિય પ્રક્રિયા માટે થયેલ કાર્ય ગણો.
View Solution
