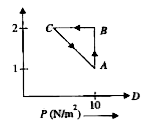
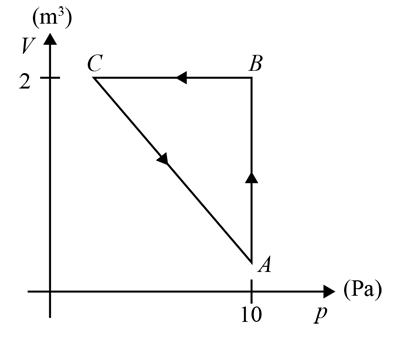
An ideal gas is taken through cycle A→B→C−A, as shown in Fig. IF the net heat supplied to the gas in the cycle is 5 J, the work done by the gas in the process C→A is
−5J
−10J
−15J
−20J
The correct Answer is:A
For cyclic process,
Qcyclic=WAB+WBC+WCA
=10J+0+WCA=5J⇒WCA=−5J
Topper's Solved these Questions
- ARCHIVES 1 VOLUME 6Book:CENGAGE PHYSICS ENGLISHChapter:ARCHIVES 1 VOLUME 6Exercise:Multi Correct
Explore 13 Videos
- ARCHIVES 1 VOLUME 6Book:CENGAGE PHYSICS ENGLISHChapter:ARCHIVES 1 VOLUME 6Exercise:Assertion-Reasoning
Explore 1 Video
- ARCHIVES 1 VOLUME 6Book:CENGAGE PHYSICS ENGLISHChapter:ARCHIVES 1 VOLUME 6Exercise:True/False
Explore 7 Videos
- ARCHIVES 2 VOLUME 6Book:CENGAGE PHYSICS ENGLISHChapter:ARCHIVES 2 VOLUME 6Exercise:Integer
Explore 4 Videos
Similar Questions
For an ideal gas
View SolutionStatement-1: The total translational kinetic energy of fall the molecules of a given mass of an ideal gas is 1.5 times the product of its pressure and its volume because.
Statement-2: The molecules of a gas collide with each other and the velocities of the molecules change due to the collision.
View SolutionThe root-mean square speeds of the molecules of different ideal gases, maintained at the same temperature are the same.
View SolutionA 20cm long string, having a mass of 1.0g, is fixed at both the ends. The tension in the string is 0.5N. The string is into vibrations using an external vibrator of frequency 100Hz. Find the separation (in cm) between the successive nodes on the string.
View SolutionAn ideal gas is taken through the cycle A→B→C→A, as shown in the figure, If the net heat supplied to the gas in the cycle is 5J, the work done by the gas in the process CtoA is
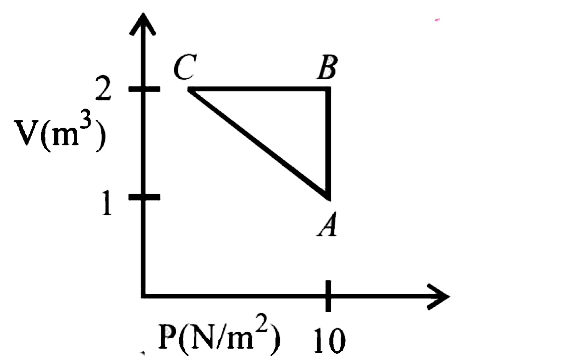
View SolutionA gas is taken through the cycle A→B→C→A, as shown in figure, what is the net work done by the gas?
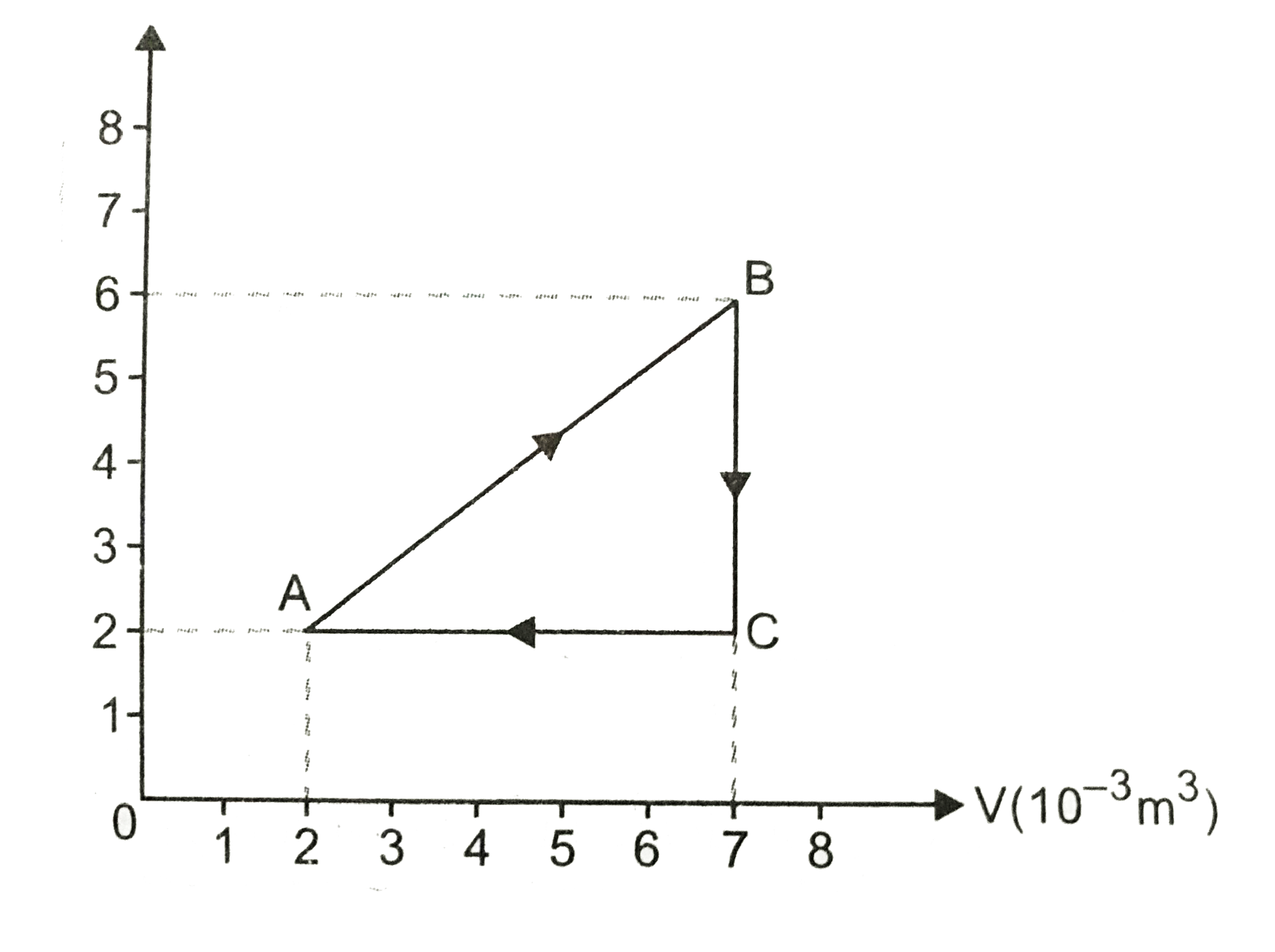
View SolutionAn ideal gas is taken through the cycle A→B→C→A As shown in figure. If net heat supplied to the gas in the cycle is 5j. Find the work done by the gas in the process C→A in Joule (taken mole value)
View SolutionA gas is taken through the cycle A to B to C to A, as shown. What is the net work done by the gas?
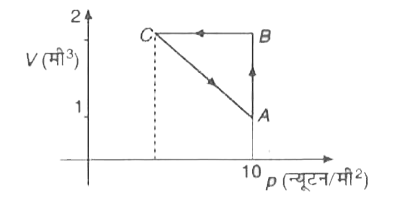
View Solutionएक आदर्श गैस को चित्रानुसार चक्र ABCA के अनुदिश से लाया जाता है | चक्र में गैस को दी गई कुल ऊष्मा 5 जूल है | प्रक्रम C→A में गैस द्वारा किया गया कार्य है
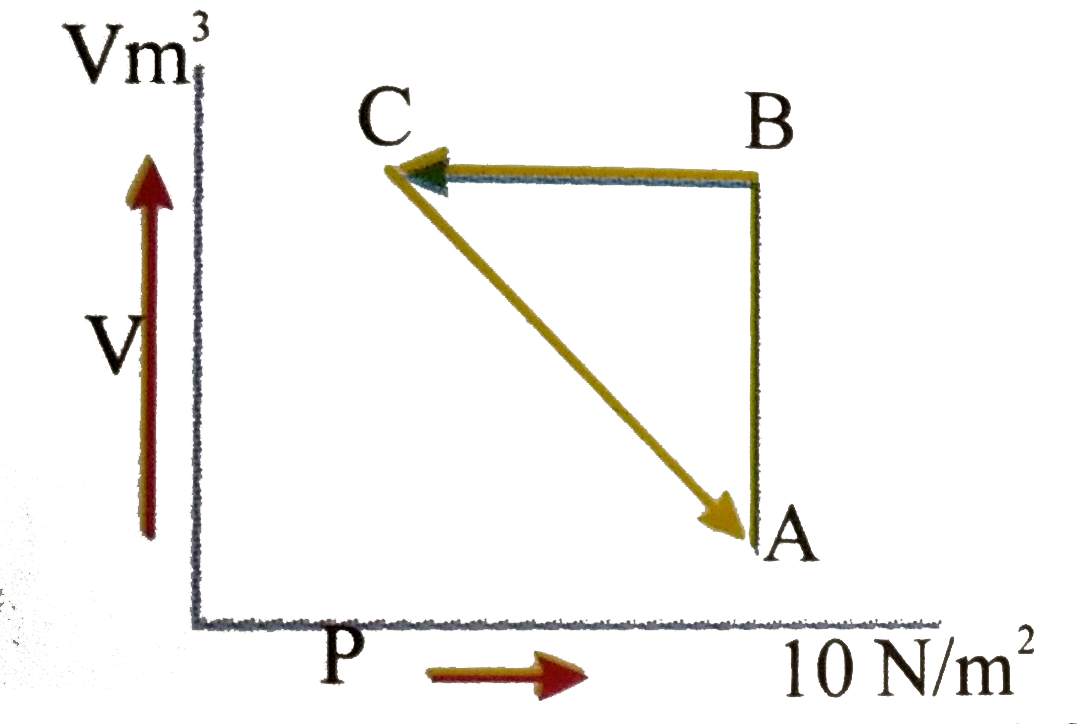
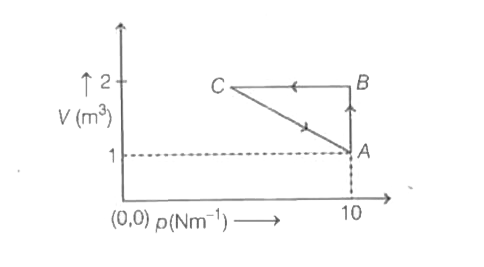
View SolutionAn ideal gas is taken through the cycle A→B→C→A as shown in the figure . If the net heat supplied to the gas in the cycles is 5J. The magnitude of word done during the process C→A is
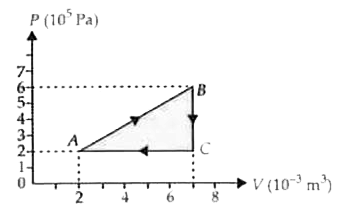
View SolutionAn ideal gas is taken in a cyclic process as shown in the figure. Calculate (a) work done by the gas. (b) work done on the gas (c ) Net work done in the process
View SolutionAn ideal gas is taken through the cycle A rarr B rarr C rarr A , as shown in figure. If the net heat supplied to the gas in the cycle is 5J, the work done by the gas in the process C rarr A is
View SolutionA gas is taken through the cycle A→B→C→A, as shown. What is the net work done by the gas ?
View SolutionAn ideal gas is taken through the cycle AtoBtoCtoA , as shown in the figure. If the net heat supplied to the gas in the cycle is 5 J, the work done by the gas in the process CtoA is
View SolutionAn ideal gas is taken through the cycle A rarr B rarr C rarr A , as shown in the figure. If the net heat supplied to the gas in the cycle is 5 J, what is the work done by the gas in the process C rarr A
View SolutionAn ideal gas is taken through the cycle A→B→C→A as shown in figure. If net heat supplied to the gas in the cycle is + 5 J, what is the work done (in J) by the gas in the process C→A?
View Solution
