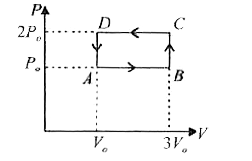
An ideal gas underoges cyclic process of ABCDA as shown in Given P−V diagram (figure)
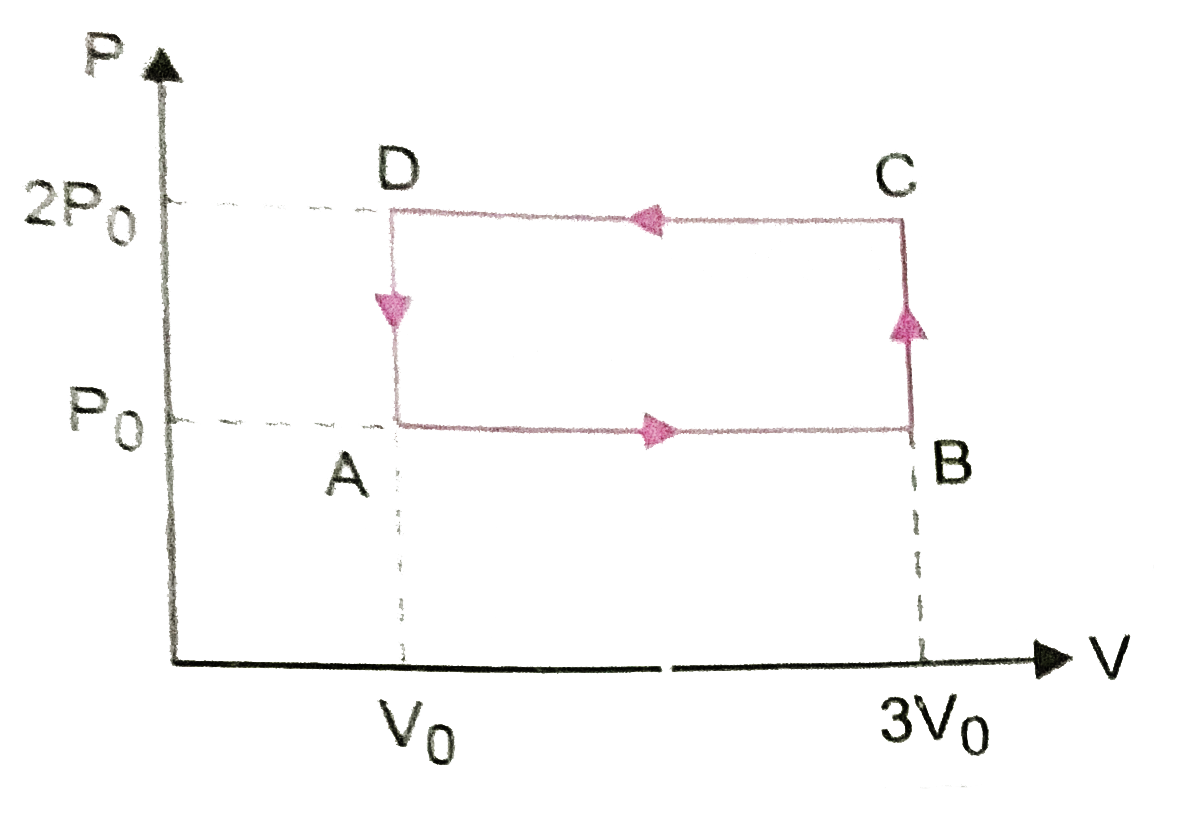
The amount of work done by the gas is
6P0V0
−2P0V0
+2P0V0
+4P0V0
The correct Answer is:B
Work done by the gas is equal to area of rectangle ABCDA, (figure).
=AB×BC=(2V0)P0=2P0V0
As the trace is anticlockwise, this work is done on the gas.
∴ Work done by the gas =−2P0V0
Similar Questions
P-V diagram of an ideal gas is as shown in figure. Work done by the gas in the process ABCD is
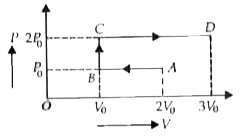
View SolutionAn ideal gas undergoes cyclic process ABCDA as shown in givend p-V diagram. The amount of work done by the gas is
View Solutionएक आदर्श गैस के चित्रानुसार दिये गये चक्रीय प्रक्रम में गैस द्वारा किया गया कार्य है :
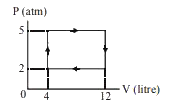
View SolutionAn ideal gas undergoes cyclic process ABCDA as shown in given p-V diagram. The amount of work done by the gas is
View Solutionकोई आदर्श गैस चित्र 12.4 के PV आरेख में दर्शाए अनुसार चक्रीय प्रक्रिया ABCDA करती है। गैस द्वारा किए गए कार्य की मात्रा है
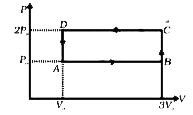
View Solutionएक आदर्श गैस दिये गये P -V ग्राफ में दर्शाए गए अनुसार चक्रीय प्रक्रम ABCDA से होकर गुजरती है। गैस द्वारा किये गये कार्य की मात्रा क्या है?
View SolutionAn ideal monoatomic gas is taken round the cycle ABCDA as shown in the P-V diagram. Compute the work done in this process.
View SolutionAn ideal gas undergoes cyclic process ABCDA as shown in given P-V diagram. The amount of work done by the gas is
View Solution
