Calculate the number of degrees of freedom of molecules of hydrogen in 1 cc of hydrogen gas at NTP.
Step by step video & image solution for Calculate the number of degrees of freedom of molecules of hydrogen in 1 cc of hydrogen gas at NTP. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- HEATBOOK - SL ARORACHAPTER - HEATEXERCISE - Problem
14 Videos
- HEATBOOK - SL ARORACHAPTER - HEATEXERCISE - Problem For Self Practice
72 Videos
- HEATBOOK - SL ARORACHAPTER - HEATEXERCISE - TYPE F
1 Video
- GRAVITATIONBOOK - SL ARORACHAPTER - GRAVITATIONEXERCISE - m
1 Video
- IIT SUPPLEMENTBOOK - SL ARORACHAPTER - IIT SUPPLEMENTEXERCISE - example
14 Videos
Similar Questions
A gas thermometer is used as a standard thermometer for measurement of temperature. When the gas container of the thermometer is immersed in water at its triple point 273.16K, the pressure in the gas thermometer reads 3.0×104N/m2. When the gas container of the same thermometer is immersed in another system, the gas pressure reads 3.5×104N/m2. The temperature of this system is therefore __________.∘C.
View SolutionA faulty thermometer reads 5∘C melting ice and 99∘C in steam. Find the correct temperature in .∘F when this faulty thermometer reads 52∘C.
View SolutionWhat amount of heat must be supplied to 2×10−2Kg of nitrogen at room temperature to rise its temperature by 45∘C at constant pressure? Given molecular mass of nitrogen is 28 and R=8.3Jmole−1K−1
View SolutionAn artificial satellite moving in a circular orbit around the earth has a total energy E0. Its potential energy is
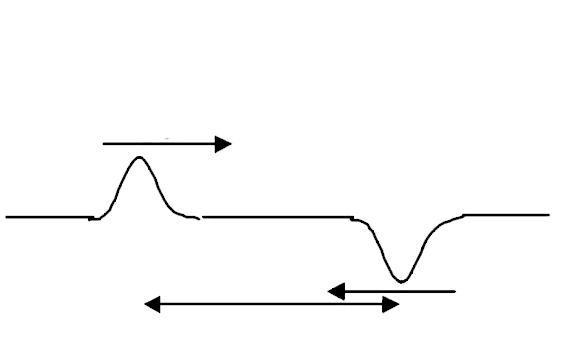
View SolutionTwo pulse in a stretched string whose centers are initially 8cm apart are moving towards each other as shown in the figure. The speed of each pulse is 2cm/s. After 2seconds, the total energy of the pulse will be
View SolutionCalculate the number of degrees of freedom of molecules of hydrogen in 1 cc of hydrogen gas at NTP.
View SolutionCalculate the total number of degree of freedom possessed by 10 c.c. of hydrogen gas at N.T.P
View SolutionCalculate the number of degrees of freedom of molecules of hydrogen in 1cc of hydrogen gas at NTP.
View SolutionIf the density of hydrogen gas at N.T.P. is 8.93×10−5gm/cm3 then the rms speed of the molecules of the hydrogen gas at NTP will be
View SolutionThe number of degrees of freedom of molecules of argon gas is
View SolutionCalculate the total number of degress of freedom possessed by the molecules in one cm^3 of H_2 gas at NTP .
View SolutionThe number of degrees of freedom for a diatomic gas molecule is …………….
View SolutionIf the density of a gas at NTP is 1.3mg//cm^(3) and velocity of sound in it is 330 m/s. The number of degree of freedom of gas molecule is
View Solution1 ml of hydrogen gas at NTP contains molecules equal to
View SolutionCalculate the number of degrees of freedom of molecules of hydrogen in 1cc of hydrogen gas at NTP.
View SolutionThe number of molecules of hydrogen in 1 cc of hydrogen gas at NTP.
View Solution

