DEVIATION FROM IDEAL BEHAVIOUR
Step by step video solution for DEVIATION FROM IDEAL BEHAVIOUR by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Deviation of real gases from ideal behaviour can be studied by plots of compressibility factor (Z) vs p. The copressibility factor is
Z=pVnRT
The compressibility factor for 1 mole of a gas obeying van der Waals gas equation at 0∘C and 100 atm pressure is found to be 0.5. The van der Waals gas equation is
(pan2V2)(V−nb)=nRT
For gases like H2andHe, which show only positive deviation from ideal behaviour, the compressibility factor is greater than 1. Is the statement true or false?
View SolutionReal Gas Analysis | Deeviation from Ideal Behaviour
View Solution
Knowledge Check
The real gases show deviation from ideal gases donot follow Boyle's law, Charles law and Avogadro law perfectly under all conditions. The deviations from ideal behaviour can be measured in terms of compressibility factor, Z which may be defined as :
Z=pVnRT
It has been observed that Z has values greater than and less than one for different gases. The behaviour of some common gases is shown here.
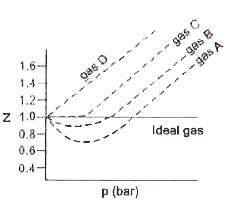
If Vo is the observed volume of a gas and Vi is the ideal gas volume, then Z isAVo−ViBVo/ViCVi/VoDVi−VoThe deviations from ideal gas behaviour PV=RT were successfully explained by van der Waals he pointed out that it is not advisable to neglect the volume of molecules and attractions between themselves at all the conditions He proposed volume correction and pressure correction as (V−b)and(P+aV2) for 1 mole of gas in ideal gas equation .
For a real gas PV>RT at all pressure ranges then .AThe gas is less compressibleBThe gas is highly compressibleCThe gas is not compressed at allDThe gas is liquefied easilyAssertion : For a certain fixed amount of gas the product PV is always constant
Reason : Real gases deviate from ideal behaviour of low pressure and high temperatureABoth A and R are true and R is the correct explanation of ABBoth A and R are true but R is not a correct explanation of ACA is true but R is falseDA is false but R is true
Ideal And Non Ideal Solution|Ideal Solutions|Non-Ideal Solutions|Non Ideal Solution With Positive Deviation|Non Ideal Solution With Negative Deviation|Summary
View SolutionColumn IColumn II(A) Carbon tetrachloride + Toluene(p) Shows positive deviation from ideal behaviour(B) Chloroform + Benzene(q) Shows negative deviation from ideal behaviour(C) Carbon tetrachloride + Chloroform (r) Mixing is endothermic (D) Benzene + Toluene(s) Shows ideal behaviour
View SolutionOut of CO2 and NH3 gases, which is expectd to show more deviation from the ideal gas behaviour ?
View SolutionGases like CO2 and CH4 show more deviation from the ideal gas behaviour as compared to gases like H2 and He, Explain
View SolutionUnder which of the following sets of conditions is a real gas expected to deviate from ideal behaviour?
(I) High pressure, small volume
(II) High temperature , low pressure
(III) Low temperature, high pressure
View Solution
