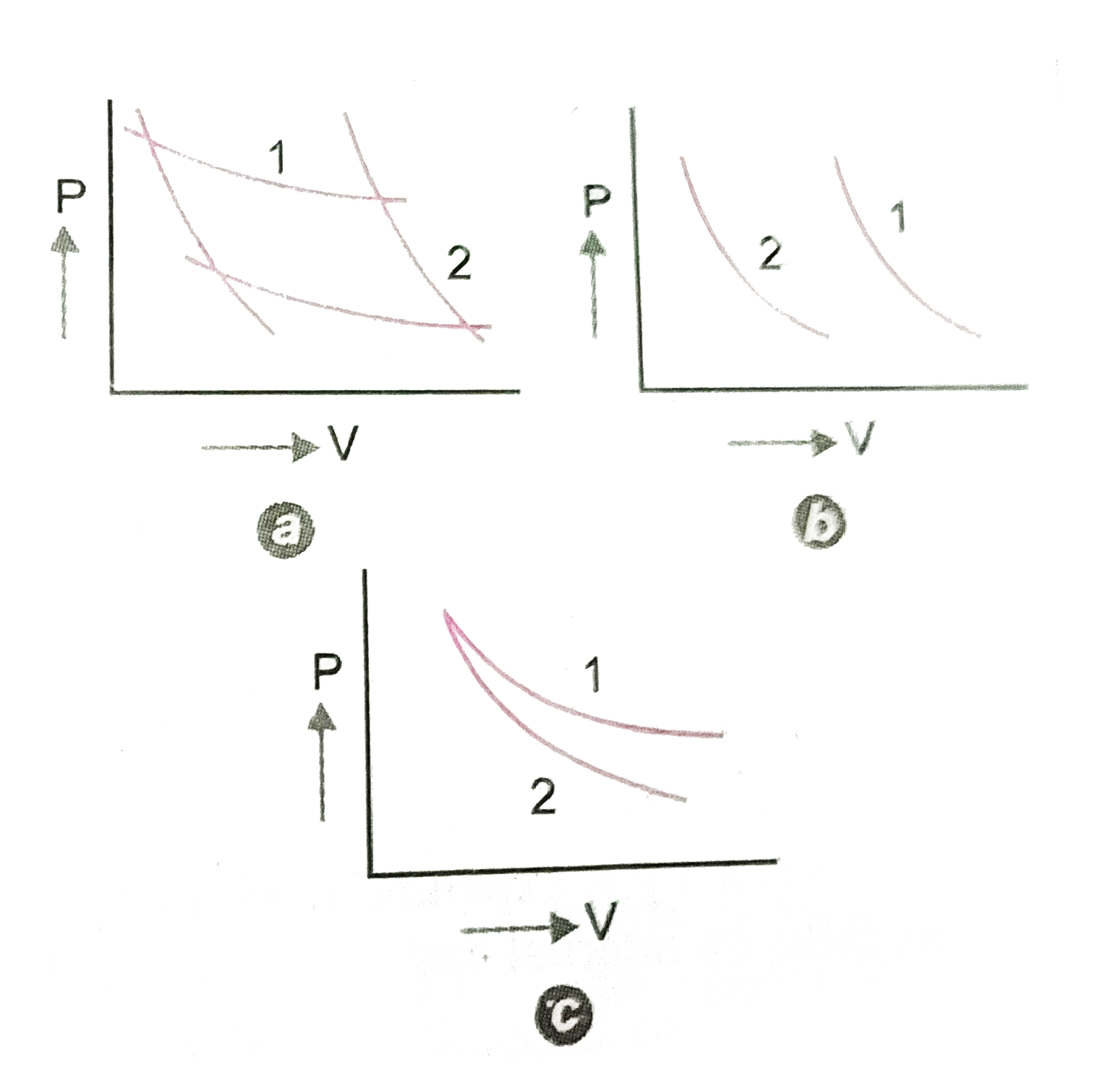
Discuss work done in an isothermal/adiabatic process in terms of indicator diagram.
Video Solution
|
Answer
Step by step video & image solution for Discuss work done in an isothermal/adiabatic process in terms of indicator diagram. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Work done in an isothermal process at constant pressure is
A2.303RTlog10V2V1B2.303RTlog10V1V2CpextΔvDPdvWhat is the slope for an isothermal process in PV indicator diagram -
APVB−PVCzeroD∝Work done in reversible isothermal process is given by
A−2.303 nRT log V2V1BnRy−1(T2−T1)C−2.303 nRt log V2V1D+2.303 nRt log V2V1