During summers in india, one of the common practice to keep cool is to make ice balls of crushed ice, dip it in flavored sugar syrup and sip it. For this a stick is inserted into crushed ice and is squeezed in the palm to make it into the ball. Equivalently in winter in those areas where it snows, people make snow balls and throw around. Explain the formation of ball out of crushed ice or snow in the light of P - T diagram of water.
Refer to the p-T diagram of water and double headed arrow. Increasing pressure at 0∘C and 1 atm takes ice into liquid state and decreasing pressure in liquid state at 0∘C and 1 atm takes water to ice state.
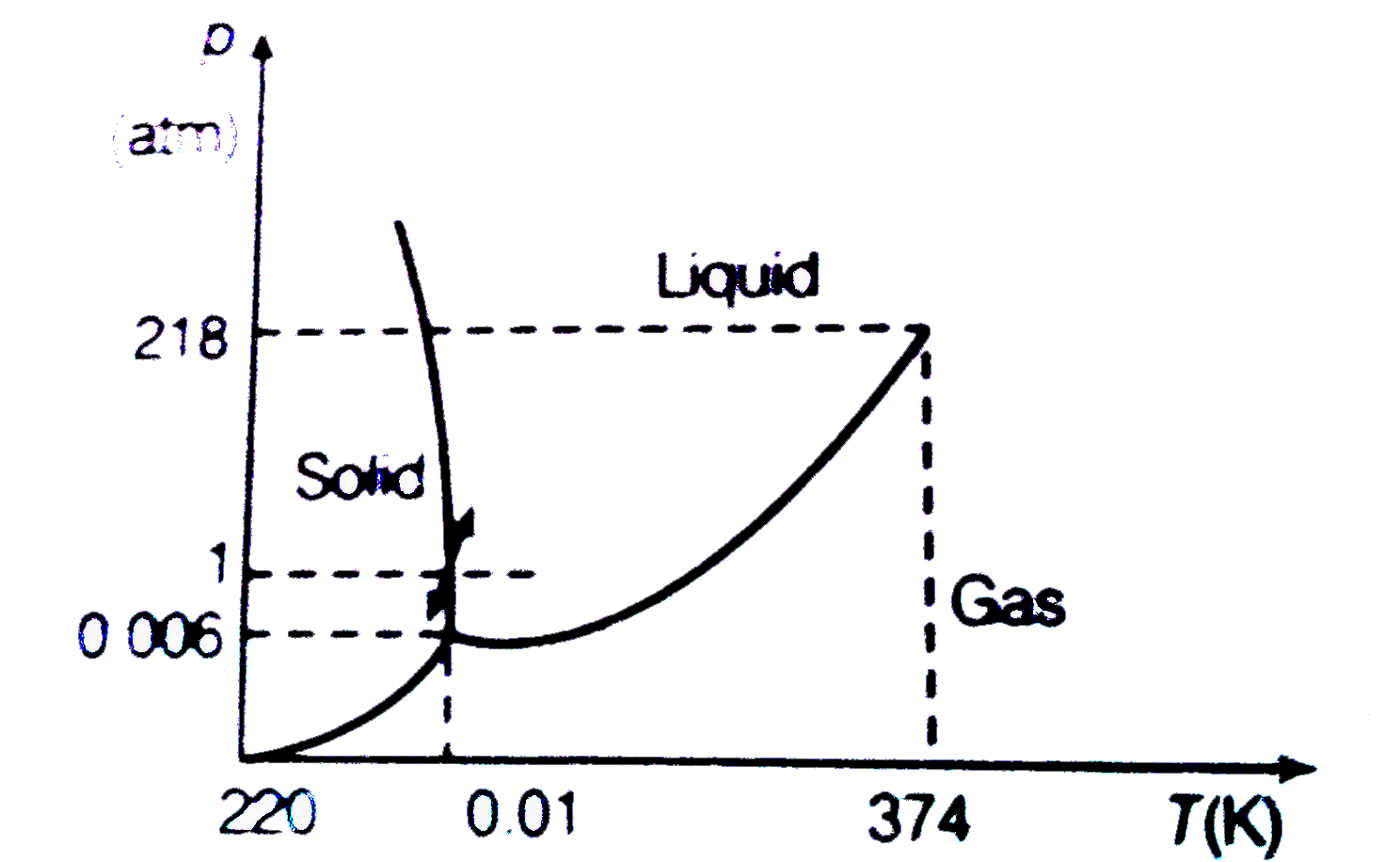
When crushed ice is squeezed, some of it melts, filling up gap between ice flakes upon releasing pressure. This water freezes, binding all ice flakes and making the ball more stable.
Topper's Solved these Questions
- THERMAL PROPERTIES OF MATTERBook:NCERT EXEMPLARChapter:THERMAL PROPERTIES OF MATTERExercise:Very short Answer type Questions
Explore 15 Videos
- SYSTEM OF PARTICLES AND ROTATIONAL MOTION Book:NCERT EXEMPLARChapter:SYSTEM OF PARTICLES AND ROTATIONAL MOTION Exercise:very short answer type questions
Explore 18 Videos
- THERMODYNAMICS Book:NCERT EXEMPLARChapter:THERMODYNAMICS Exercise:LONG ANSWER TYPE QUESTIONS
Explore 5 Videos
Similar Questions
Is the bulb of a thermoeter made of diathermic or adiabatic wall ?
View Solutionthe centre of gravity of a body on the earth coincides with its centre of mass for a small object whereas for an extended object it may not ,what is the qualitaitve meaning of small and extended in this regard ?
for which of the following two coincides ? A building , a pond , a lake ,a mountain ?
View SolutionConsider a P−V diagram in which the path followed by one mole of perfect gas in a cyclinderical container is shown in (figure)
(a) Find the work done when the gas is taken from state 1 to state 2.
(b) What is the ratio of temperatures T1/T2,ifV2=2V1?
(c ) Given the internal energy for one mole of gas at temperature Tis(3/2)RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2=2V1.
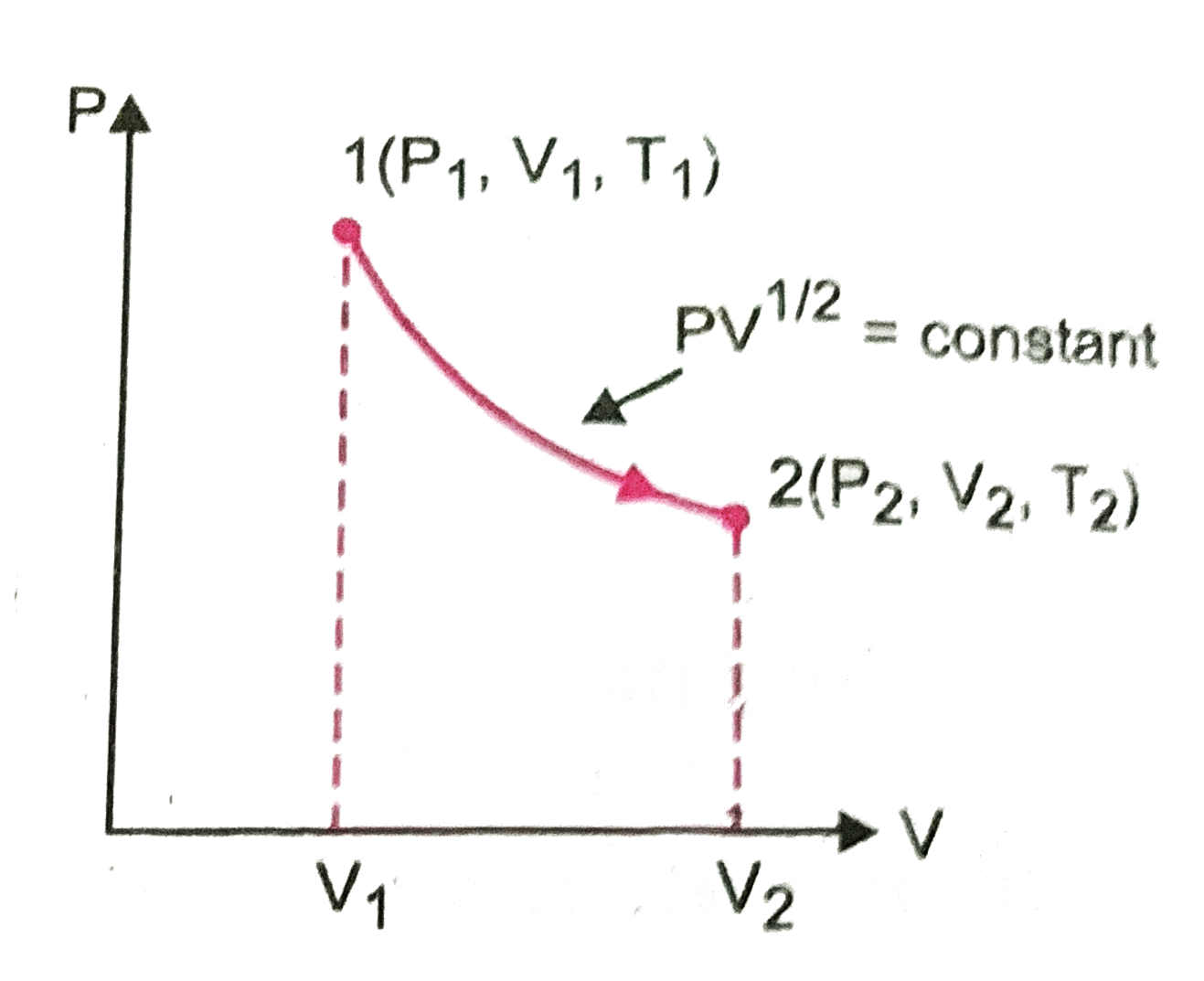
View SolutionWhy snow is better heat insulator than ice?
View SolutionDuring summers in india, one of the common practice to keep cool is to make ice balls of crushed ice, dip it in flavoured suger syrup and sip it. For this a stick is inserted into crushed ice and is squeezed in the palm to make it into the ball. Equivalently in winter in thouse ares where it snows, people make snow balls and throw around. Explain the formation of ball out of crushed ise or snow in the light of P - T diagram of water.
View Solutionबर्फ का गलनांक निकालने के लिए चूर किए गए बर्फ को रखा जाता है
View Solutionif crushed ice is pressed and then the pressure is released, a lump of ice is formed.
View SolutionIf crushed ice is pressed and then the pressure is released, a lump of ice is formed. Why does this happen? Explain with reference to regelation.
View SolutionHow does the shredded ice convert into solid ice ball (during the preparation of an ice ball)?
View SolutionWhile making ice-creams in metal or plastic cones, the ice-cream seller puts a mixture of ice and common salt around the cones and not ice alone. Why?
View SolutionHow does the shredded ice convert into solid ice ball (during the preparation of an ice ball)?
View Solutionकेवल दूध से आइसक्रीम बनाने की तुलना में शकरा युक्त दूध से आइसक्रीम बंनाने में अधिक समय लगता है क्यों
View SolutionExplain the process of making ice cream
View SolutionThe plains characterised by snow, ice and frozen soil during most of the year are known as
View Solutionबर्फ में थोड़ी भी अशुद्धि से बर्फ का गलनांक
View SolutionDuring summers in India, one of the common practice to keep cool is to make ice balls of crushed ice, dip it in flavoured sugar syrup and sip it. For this a stick is inserted into crushed ice and is squeezed in the palm to make it into the ball. Equivalently in winter, in those areas where it snows, people make snow balls and throw around. Explain the formation of ball out of crushed ice or snow in the light of P - T diagram of water.
View Solution
