Explain the experiment of measuring the real and apparent expansion of a liquid with a neat diagram.Solution in Tamil
Video Solution
Text Solution
When the temperature of a gas is kept constant, the volume of a fixed mass of gas is inversely proportional to its pressure.
P∝1/V
(ii) (i) To start with, the liquid whose real and apparent expansion is to the determined is poured in a container up to a level. Mark this level as L1.
(ii) Now, heat the container and the liquid using a burner as shown in the fiugre Initially, the container receives the thermal energy and it expands.
(iii) As a result the volume of the liquid appeares to have reduced. Mark this reduced level of liquid as L2.
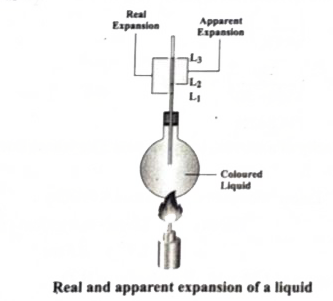
On further heating, the thermal energy supplied to the liquid through the container results in the expansion of the liquid. Hence , the level of liquid rises to L3. Now, the difference between the levels L1andL3 is called as apparent expanision, and the difference between hte levels L2andL3 is called real expansion. The real expansion is always more than that of apparent expansion.
Real expansion = L3−L2
Apparent expansion = L3−L1.
|
Similar Questions
Explain the experiment of measuring the real and apparent expansion of a liquid with a neat diagram.
View SolutionReal expansion of a liquid is equal to the difference between apparent expansion and expansion of vessel
View SolutionExplain the experiment of measuring the real and apparent expansion of a liquid with a neat diagram.
View SolutionWith a neat diagram explain the working of G.P. Thomson's experiment.
View SolutionCoefficient of real expansion of a liquid is 0.000182/∘C. If coefficient of linear expansion of vessel is 0.000009/∘C, coefficient of apparent expansion of the liquid is
View SolutionDefine coefficient of real and apparent expansions of a liquid.
View SolutionIf the coefficient of apparent expansion of the liquid is greater than the coefficient of real expansion of the liquid, what do you infer?
View Solution
