Explain why the temperature of a gas drops in an adiabatic expansion, on basis of the kinetic theory of gases,
In an adiabaticf expansion ΔQ=0. But ΔQ=ΔU=+ΔW by the first law of thermodynamics. Therefore, in an adiabatic expanison,
ΔW=−ΔU
Thus, work down by a gas in an adiabatic expanison is associated with a decrease of internal energy. So its temperature decreasese.
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Subjective
22 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Single Correct
140 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Exercise 2.1
20 Videos
- KINETIC THEORY OF GASESBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASESEXERCISE - Compression
2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONEXERCISE - Single correct anwer type
14 Videos
Similar Questions
Five grams of helium having rms speed of molecules 1000m/s and 24g of oxygen having rms speed of 1000m/s are introduced into a thermally isolated vessel. Find the rms speeds of helium and oxygen individually when thermal equilibrium is attained. Neglect the heat capacity of vessel.
View SolutionThe temperature of a gas is raised while its volume remains constant, the pressure exerted by the gas on the walls of the container increases because its molecules
View SolutionThe average kinetic energy per molecule of all monatomic gases is the same at the same temperature. Is this ture or false ?
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
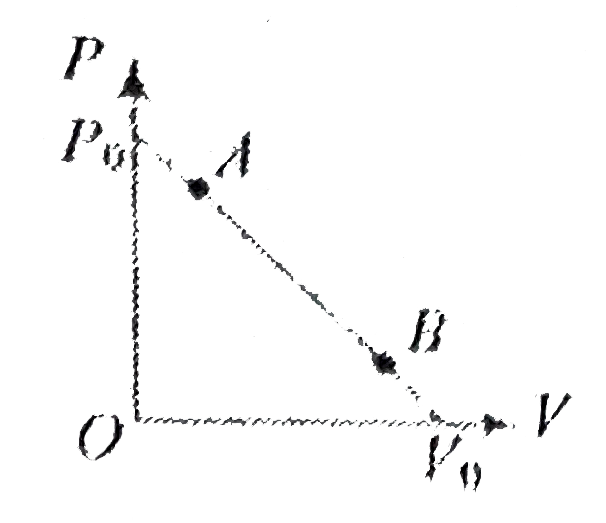
The absolute temperature T versus volume V for the given process is
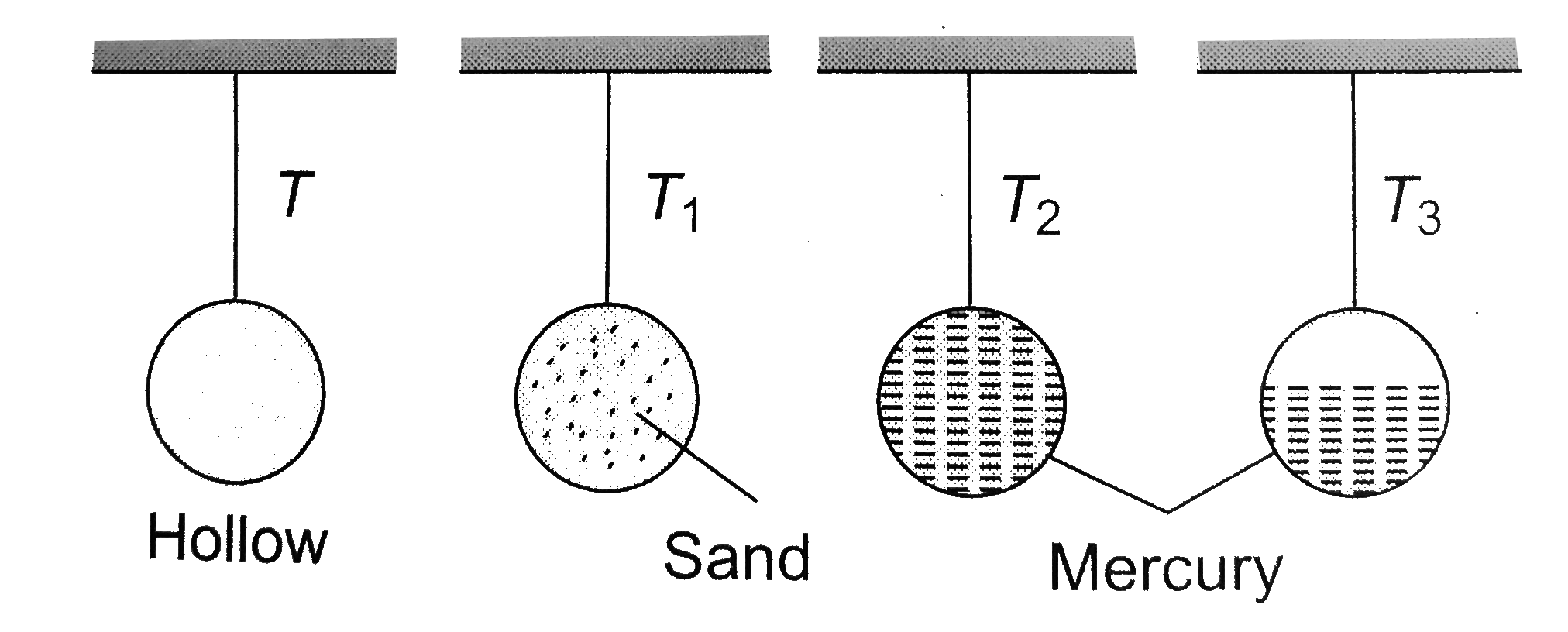
View SolutionThe period of a simple pendulum whose bob is hollow metallic sphere is T. The period is T1 when the bob is filled with sand, T2 where it is filled with mercury and T3 when it is half filled with mercury Which of the following is true?
View SolutionExplain why the temperature of a gas drops in an adiabatic expansion, on basis of the kinetic theory of gases,
View SolutionWhen a gas expands at constant temperature, why does the pressure decrease? Explanin it on the basis of kinetic theory of gases.
View SolutionWhen a gas is heated, its temperature increases. Explain it on the basis of kinetic theory of gases.
View SolutionOn the basis of kinetic theory of gases, explain how does a gas exert pressure.
View SolutionOn the basis of kinetic theory of gases, explain how does a gas exert pressure?
View SolutionExplain on the basis of kinetic theory of gases, how the pressure of the gas changes if its volume is reduced at constant temperature.
View SolutionThe theory that attempts to explain the behaviour of gases is known as kinetic molecular theory.
On the basis of this theory, explain the compressible nature of gases and the temperature dependence on kinetic energy.
View SolutionExplain on the basis of kinetic theory of gases why evaporation causes cooling.
View SolutionWhat is the interpretation of temperature on the basis of kinetic theory of gas?
View SolutionDeduce the gas laws on the basis of kinetic theory of gases.
View SolutionOn the basis of the kinetic theory explain why the pressure of a gas at constant volume increases with rise in temperature?
View Solution

