Figure shows two processes a and b for a given sample of a gas. If ΔQ1, ΔQ2 are the amounts of heat absorbed by the system in the two cases and ΔU1, ΔU2 are changes in internal energies respectively, then
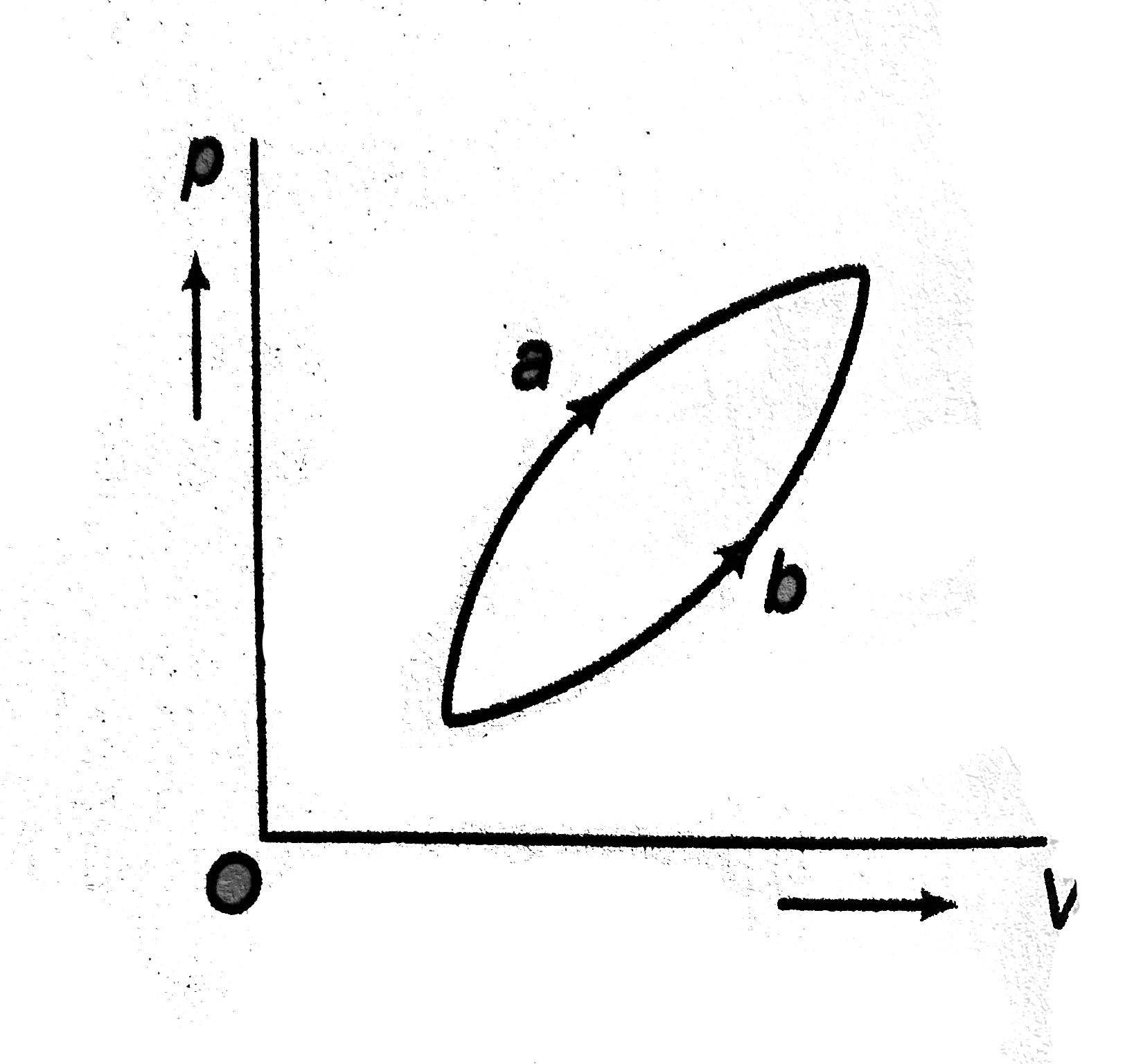
The correct Answer is:A, B
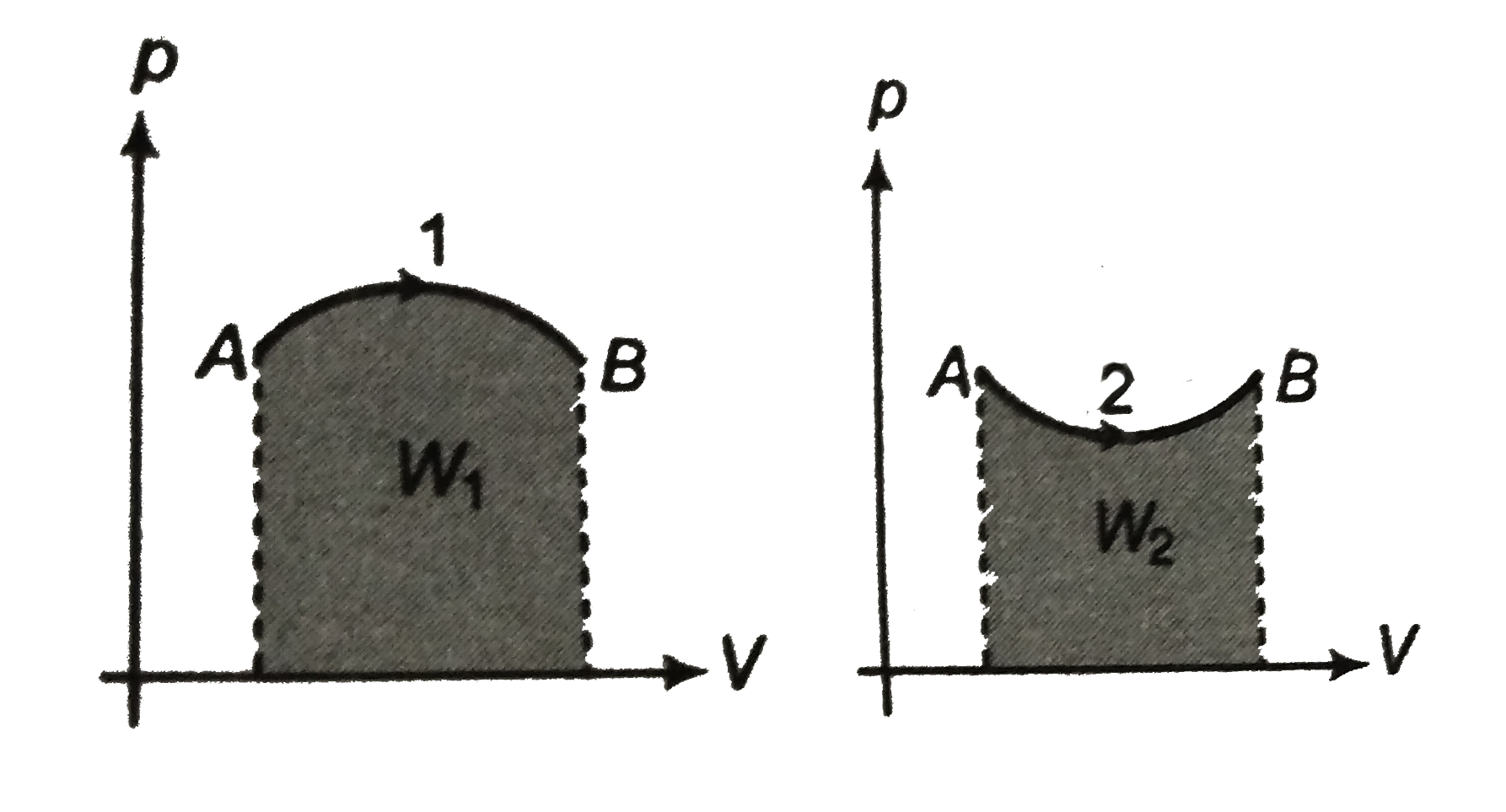
Q1=W1+ΔU1 and Q2=W2+ΔU2
U is a state function. Hence, ΔU depends only on the initial and final position. Therefore,
ΔU1=ΔU2
But, W1>W2
as the area under 1 is greater than area under 2. Hence,
Topper's Solved these Questions
- LAWS OF THERMODYNAMICSBook:DC PANDEY ENGLISHChapter:LAWS OF THERMODYNAMICSExercise:Exercise 21.4
Explore 8 Videos
- LAWS OF THERMODYNAMICSBook:DC PANDEY ENGLISHChapter:LAWS OF THERMODYNAMICSExercise:Level 1 Assertion And Reason
Explore 10 Videos
- LAWS OF THERMODYNAMICSBook:DC PANDEY ENGLISHChapter:LAWS OF THERMODYNAMICSExercise:Exercise 21.2
Explore 7 Videos
- LAWS OF MOTIONBook:DC PANDEY ENGLISHChapter:LAWS OF MOTIONExercise:Medical entrances gallery
Explore 39 Videos
- MAGNETIC EFFECT OF CURRENT AND MAGNETISMBook:DC PANDEY ENGLISHChapter:MAGNETIC EFFECT OF CURRENT AND MAGNETISMExercise:Integer type Questions
Explore 10 Videos
Similar Questions
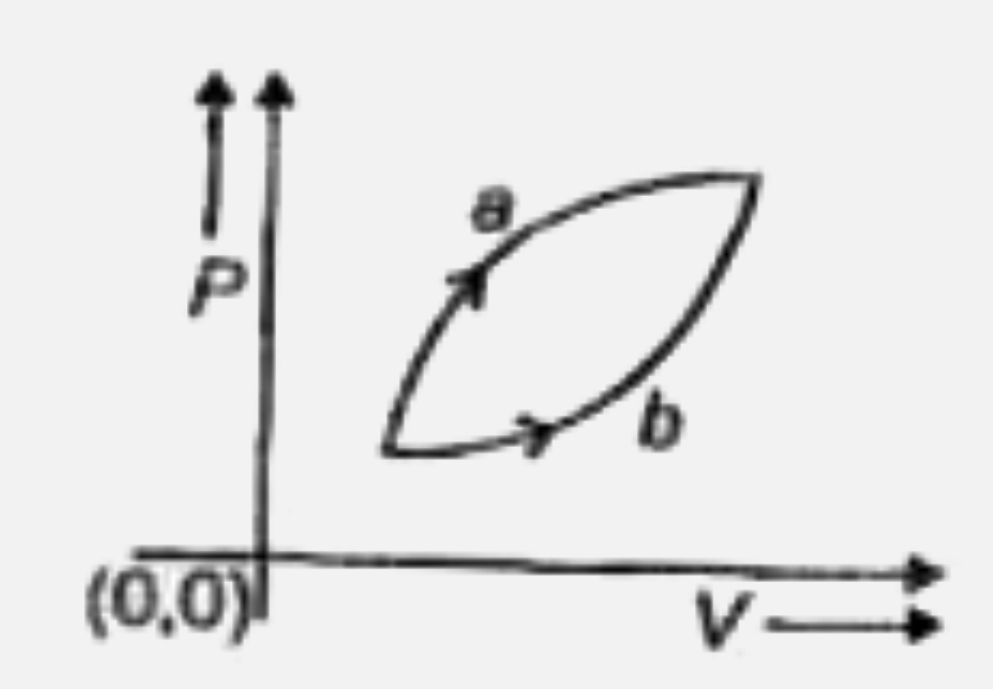
Figure shows two processes a and b for a given sample of gas. If ΔQ1,ΔQ2 are the amount of heat absorbed by the systemin the two cases , and ΔU1,ΔU2 are changes ininternal energy respeclively, then
View SolutionFollowing figure shows two process A and B for a gas. If ΔQA and ΔQB are the amount of heat absorbed by the system in two case, and ΔUA and ΔUB are changes in internal energies, respectively, then :
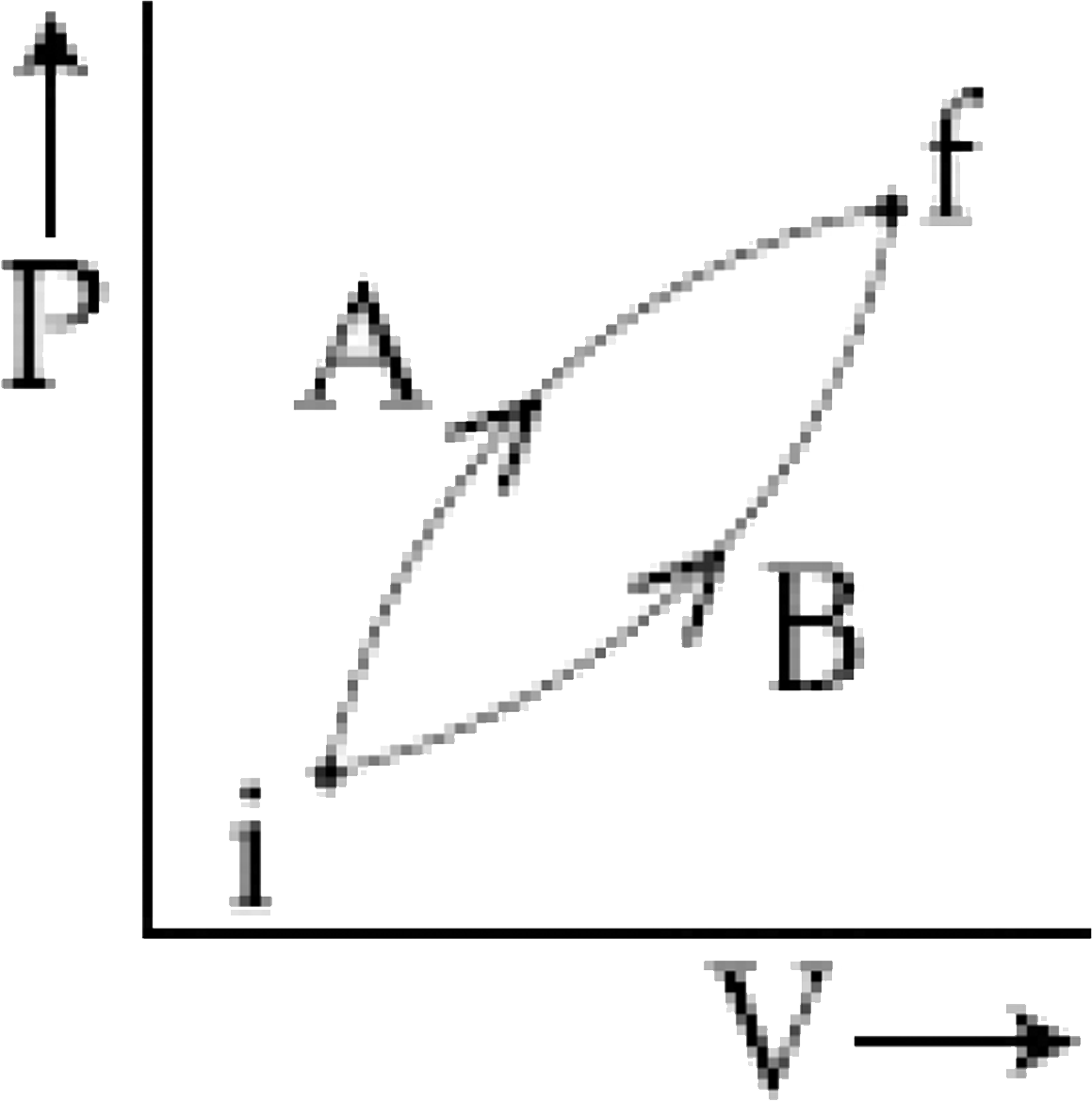
View SolutionFollowing figure shows two process A and B for a gas. If ΔQA and ΔQB are the amount of heat absorbed by the system in two case, and ΔUA and ΔUB are changes in internal energies, respectively, then :

View SolutionAn ideal gas goes from state A to state B via three different processes as indicated in the P-V diagram -
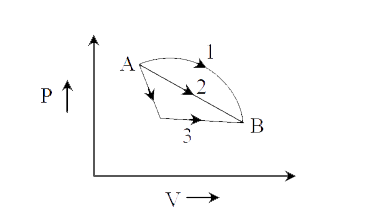
If Q1,Q2,Q3 indicate the heat absorbed by the gas along the three process and ΔU1,ΔU2,ΔU3, indicate the change in internal energy along the three processes respectively, then -
View Solution550kJcycle−1 work is done by 1 mol of an ideal gas in a cyclic process. The amount of heat absorbed by the system in one cycle is
View SolutionRefer to figure. Let ΔU1 and ΔU2 be the changes in internal energy of the system in the processes A and B. then
View SolutionA system goes from A and B via two processes. I and II as shown in figure. If ΔU1andΔU2 are the changes in internal energies in the processes I and II respectively, then
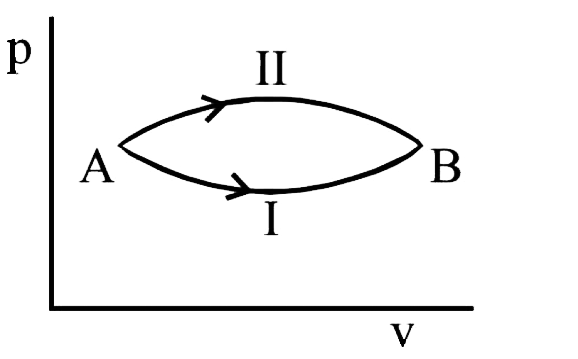
View SolutionA system goes from A and B via two processes. I and II as shown in figure. If ΔU1andΔU2 are the changes in internal energies in the processes I and II respectively, then

View SolutionA system goes from A and B via two processes. I and II as shown in figure. If ΔU1andΔU2 are the changes in internal energies in the processes I and II respectively, then

View SolutionIn a process, 701 J of heat is absorbed by a system and 394 J of work is done by the system. What is the change in internal energy of the process?
View Solution
