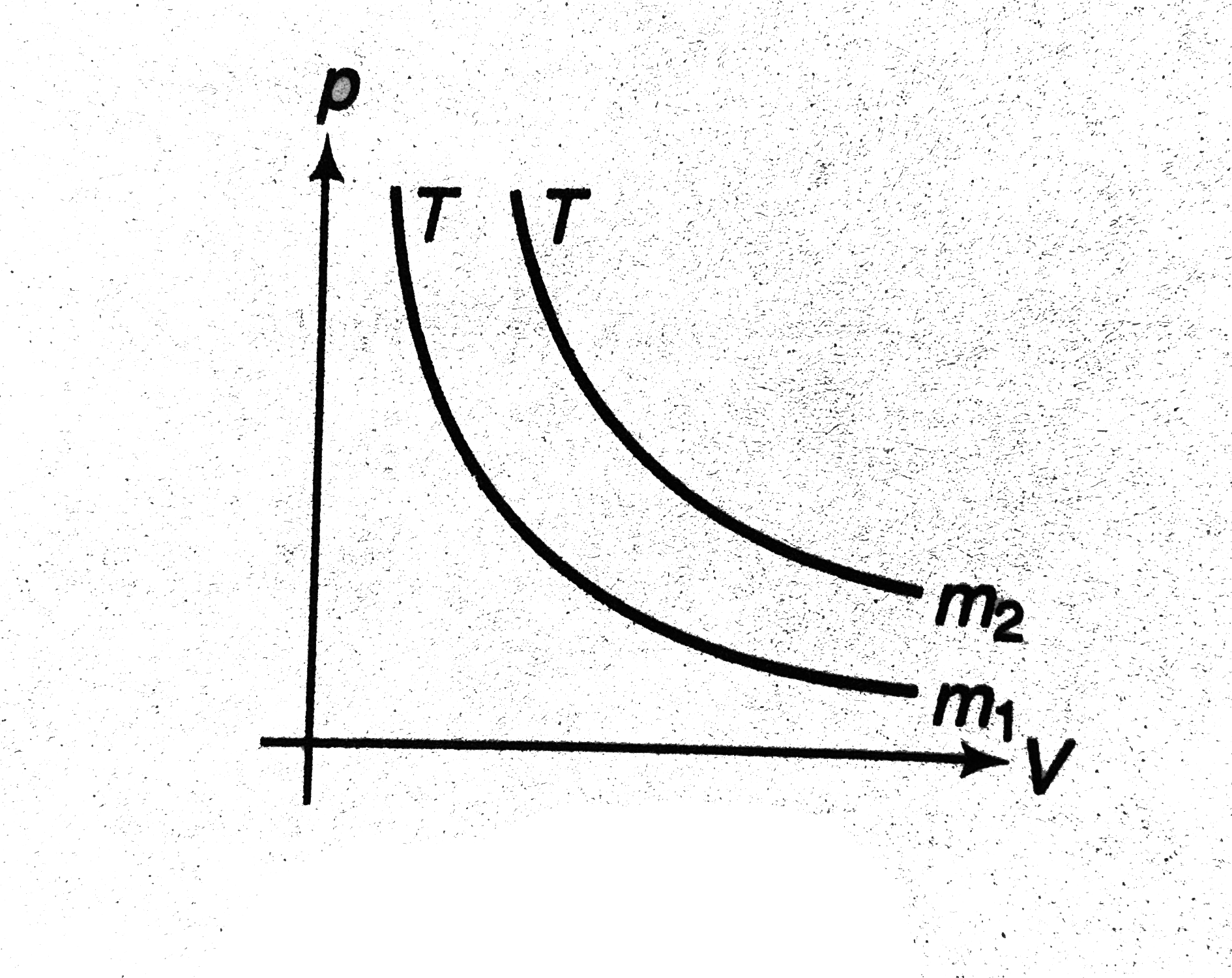
From the graph for an ideal gas, state whether m1orm2 is greater.
 .
.
The correct Answer is:A, B
pV=nRT=(mM)RT
∴T=(VMmR)p
At constant volume (T - p) graph is a straight line of
slope, = VMmR∝1m
Slope of (m1) is less. Hence, (m1) is greater.
Topper's Solved these Questions
- THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASESBook:DC PANDEY ENGLISHChapter:THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASESExercise:Exercise 20.4
Explore 3 Videos
- THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASESBook:DC PANDEY ENGLISHChapter:THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASESExercise:Exercise 20.5
Explore 7 Videos
- THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASESBook:DC PANDEY ENGLISHChapter:THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASESExercise:Exercise 20.2
Explore 7 Videos
- THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASESBook:DC PANDEY ENGLISHChapter:THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASESExercise:Medical entrance gallary
Explore 30 Videos
- UNIT AND DIMENSIONSBook:DC PANDEY ENGLISHChapter:UNIT AND DIMENSIONSExercise:Assertion And Reason
Explore 2 Videos
Similar Questions
On the P−T graph of an ideal gas, choose the correct option(s):
View SolutionState the Avogadro law of ideal gas
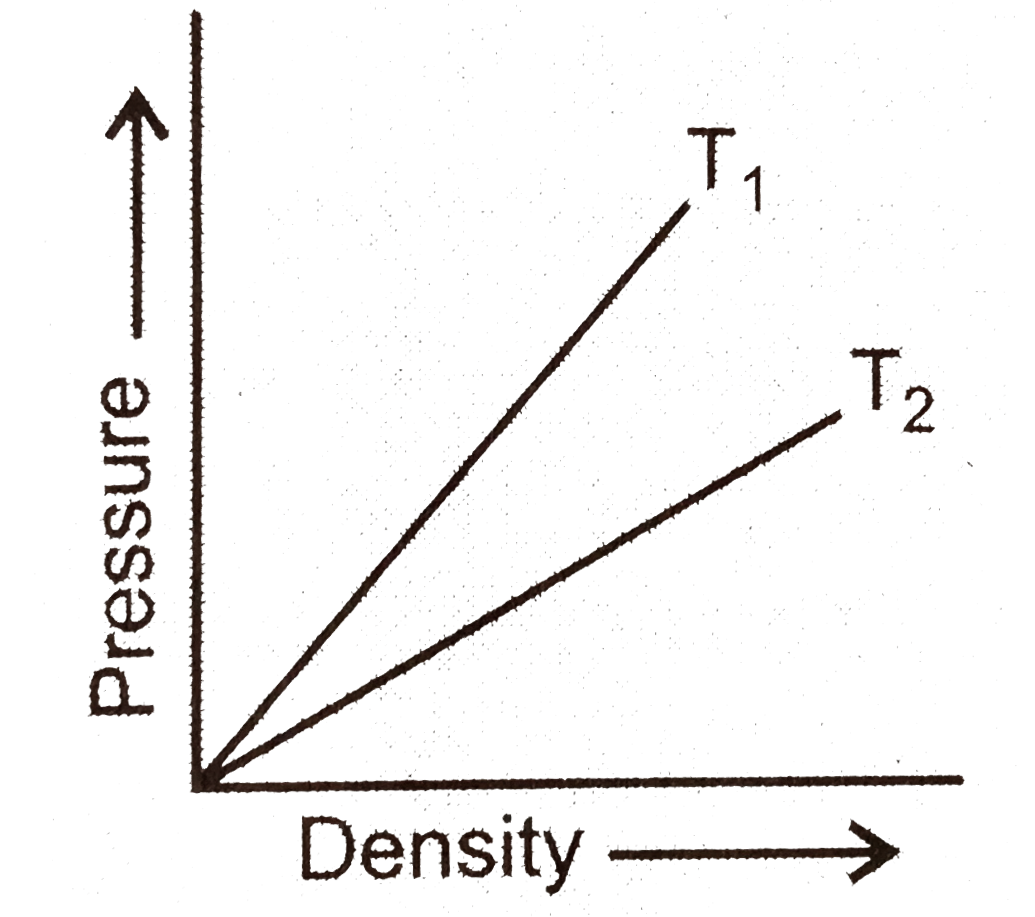
View SolutionFigure shows graphs of pressure versus density for an ideal gas at two temperatures T1 and T2 Which is correct ?
 .
.
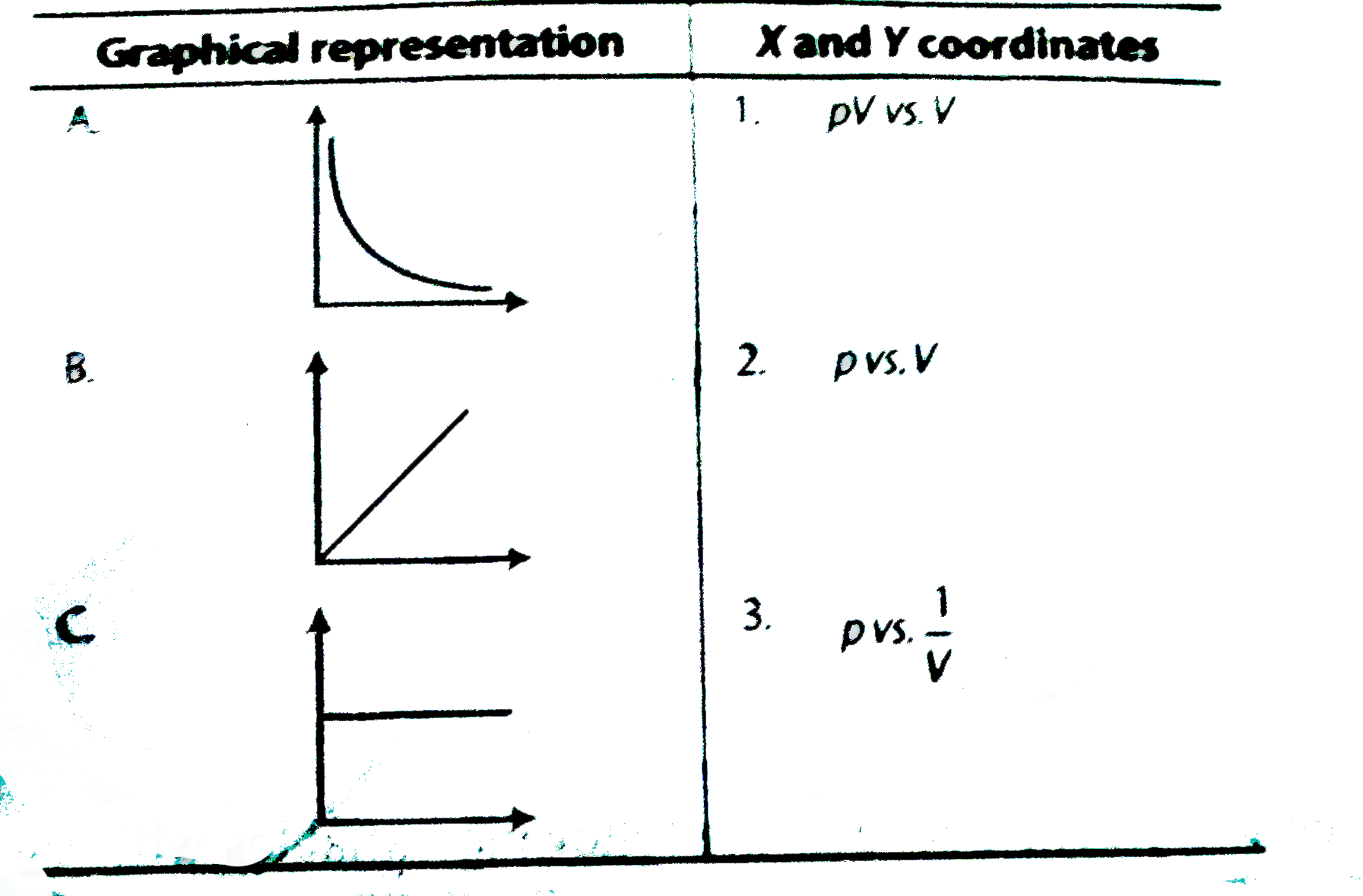
View SolutionMatch the following graphs of ideal gas with their coordinates.
View SolutionA gas in a cyclinder is held at a constant pressure of 2.30×105Pa and is cooled and compressed from 1.70m3 to 1.20m3. The internal energy of the gas decreases by 1.40×105J. (a) Find the work done by the gas. (b) Find the absolute value |Q| of the heat flow into or out of the gas and state the direction of the heat flow. (c) Does it matter whether or not the gas is ideal? Why or why not?
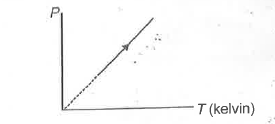
View SolutionThe graph of pressure and temperature for an ideal gas is as shown in figure. The given process is
View SolutionPressure versus density graph of an ideal gas is shown in figure
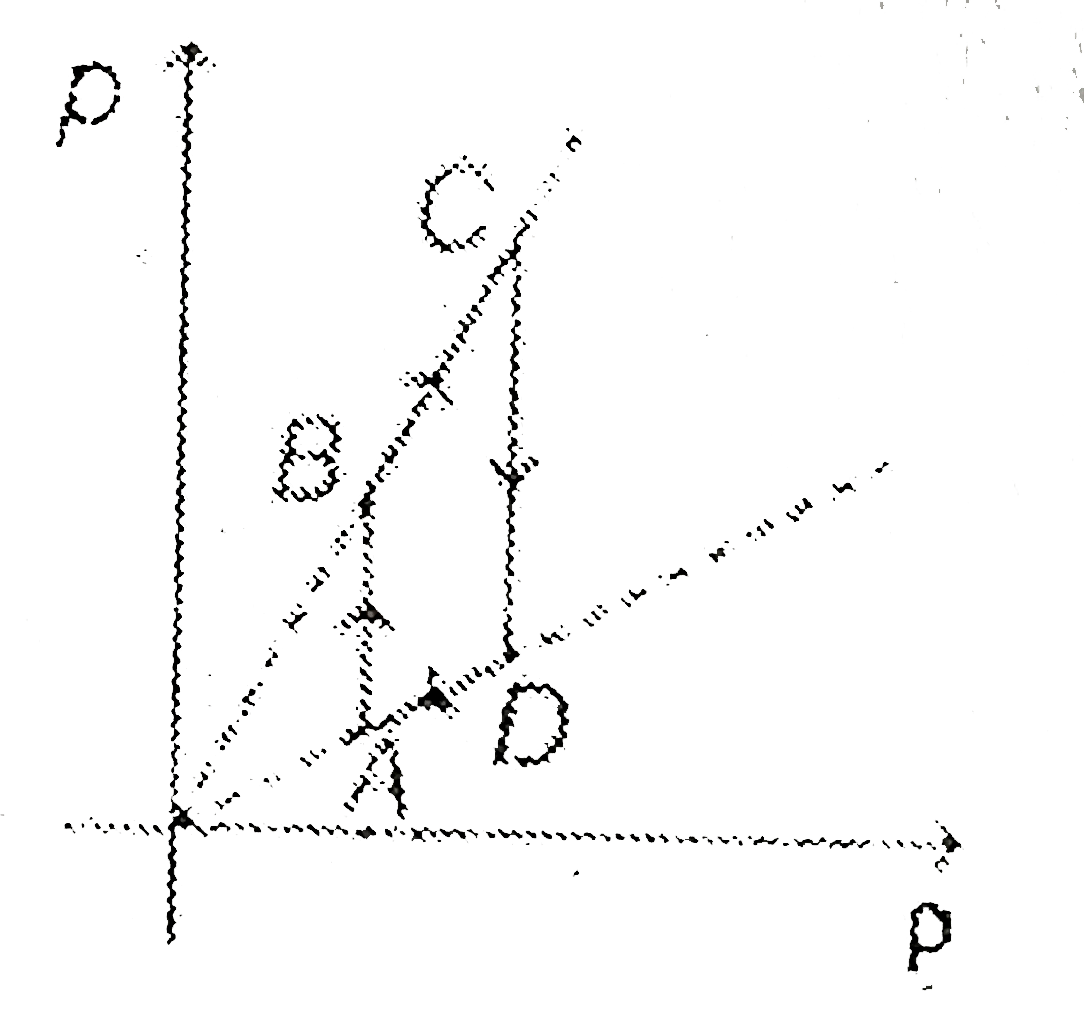
View SolutionThe p−V diagram of two different masses m1 and m2 are drawn (as shown) at constant temperature (T). State whether m1>m2orm2>m2.
 .
.
View SolutionTwo identical containers A and B having same volume of an ideal gas at same temperature have mass of the gas as m1 and m2 respectively and 2m1=3m2. The gas in each cylinder expands isomthermally to double of its volume. If change in pressure in A is 300Pa, then the change in pressure in B is
View Solutiongraph of an ideal gas is as shown in figure.
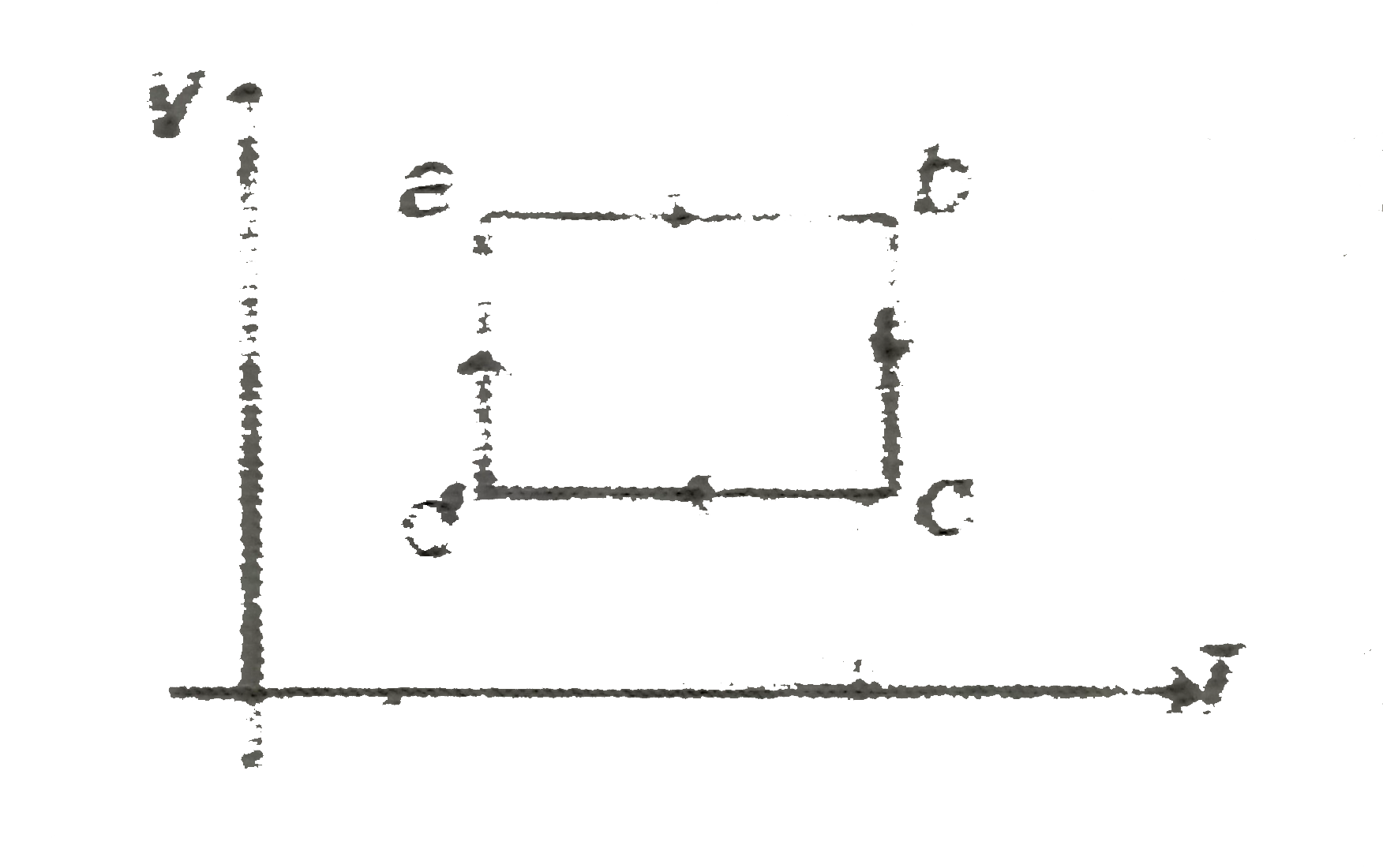
Heat is supplied to the gas in process (s).
View Solution
