If one of the two electrons of a H2 molecule is removed, we get a hydrogen molecular ion H+2. In the ground state of an H+2, the two protons are separated by roughly 1.5Å, and the electron is roughly 1Å from each proton. Determine the potential energy of the system. Specific your choice of the zero of potential energy.Solution in Tamil
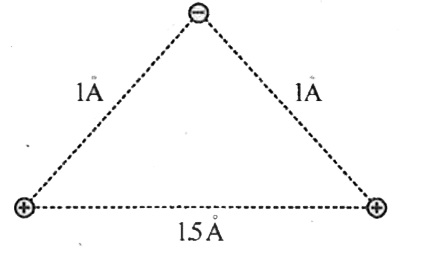
U=U1+U2+U3 U1=9×109e21.5×10−10U2=U3
U2+U3=9×109×−2e21×10−10
U=9×109e21010(11.5−21)=9×109×(1.6)2×1038×−210−10×1.5×1.6×10−19eV
=−9×1.6×21.5=−9×16×430=−19.2eV
Similar Questions
If one of the two electrons of a H2 molecule is removed, we get a hydrogen molecular ion H+2.In the ground state of an H+2 , the two protons are separated by roughly 1.5 Å, and the electron is roughly 1 Å from each proton. Determine the potential energy of the system. Specify your choice of the zero of potential energy
View SolutionIf one of the two electrons of a H2 molecule is removed, we get a hydrogen molecular ion H+2.In the ground state of an H+2 , the two protons are separated by roughly 1.5 Å, and the electron is roughly 1 Å from each proton. Determine the potential energy of the system. Specify your choice of the zero of potential energy
View SolutionIf one of the two electrons of a H2 molecule is removed, we get a hydrogen molecular ion H+2.In the ground state of an H+2 , the two protons are separated by roughly 1.5 Å, and the electron is roughly 1 Å from each proton. Determine the potential energy of the system. Specify your choice of the zero of potential energy
View SolutionIf one of the two electrons of a hydrogen molecule is removed, we get a hydrogen molecule ion (H_(2)^(+)) . In the ground state of H_(2)^(+) , the two protons are separated roughly by 1.5 Å and electron is roughly 1 Å from each proton. Determine the potential energy of the system. Specify your choice of zero of potential energy.
View SolutionIf one of the two electrons of a H2 molecule is removed, we get a hydrogen molecular ion H+2.In the ground state of an H+2, the two protons are separated by roughly 1.5 Å, and the electron is roughly 1 Å from each proton. Determine the potential energy of the system. Specify your choice of the zero of potential energy.
View SolutionIf one of the two electrons of a H2 molecule is removed, we get a hydrogen molecular ion H+2.In the ground state of an H+2 , the two protons are separated by roughly 1.5 Å, and the electron is roughly 1 Å from each proton. Determine the potential energy of the system. Specify your choice of the zero of potential energy
View SolutionIn a hydrogen atom, the electron and proton are bound together at a distance of above 0.53 Å. Estimate the potential energy of the system in eV, assuming zero potential energy at infinite separation between the electron and the proton.
View SolutionIf one of the two electrons of a H2 molecule is removed, we get a hydrogen molecular ion H+2. In the ground state of an H+2, the two protons are separated by roughly 1.5Ao, and the electron is roughly 1Ao from each proton. Determine the potential energy of the system. Specify your choice of the zero of potential energy.
View Solution
