If P is the pressure of a gas and ρ is its density , then find the dimension of velocity in terms of P and ρ .
Step by step video, text & image solution for If P is the pressure of a gas and rho is its density , then find the dimension of velocity in terms of P and rho . by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- UNIT AND DIMENSIONSBOOK - ANURAG MISHRACHAPTER - UNIT AND DIMENSIONSEXERCISE - Exercise
6 Videos
- UNIT AND DIMENSIONSBOOK - ANURAG MISHRACHAPTER - UNIT AND DIMENSIONSEXERCISE - Only one Alternative is Correct
25 Videos
- RIGID BODY MOTIONBOOK - ANURAG MISHRACHAPTER - RIGID BODY MOTIONEXERCISE - MATCH THE COLUMN
12 Videos
- WORK AND ENERGYBOOK - ANURAG MISHRACHAPTER - WORK AND ENERGYEXERCISE - Matching types problems
14 Videos
Similar Questions
In two systems of units the relation between velocity acceleration and force is given by v2=v1ε2τ,a2=a1ετ,F2=F1ετ, where ε and τ constants then find in this new system:
(a) m2m1 , (b) L2L1
View SolutionE, m, L, G denote energy mass, angular momentum & gravitation constant respectively. The dimensions of EL2m5G2 will be that of :
View SolutionMatch the statements, situations in Column I with that in Column II. One or more matching is possible.
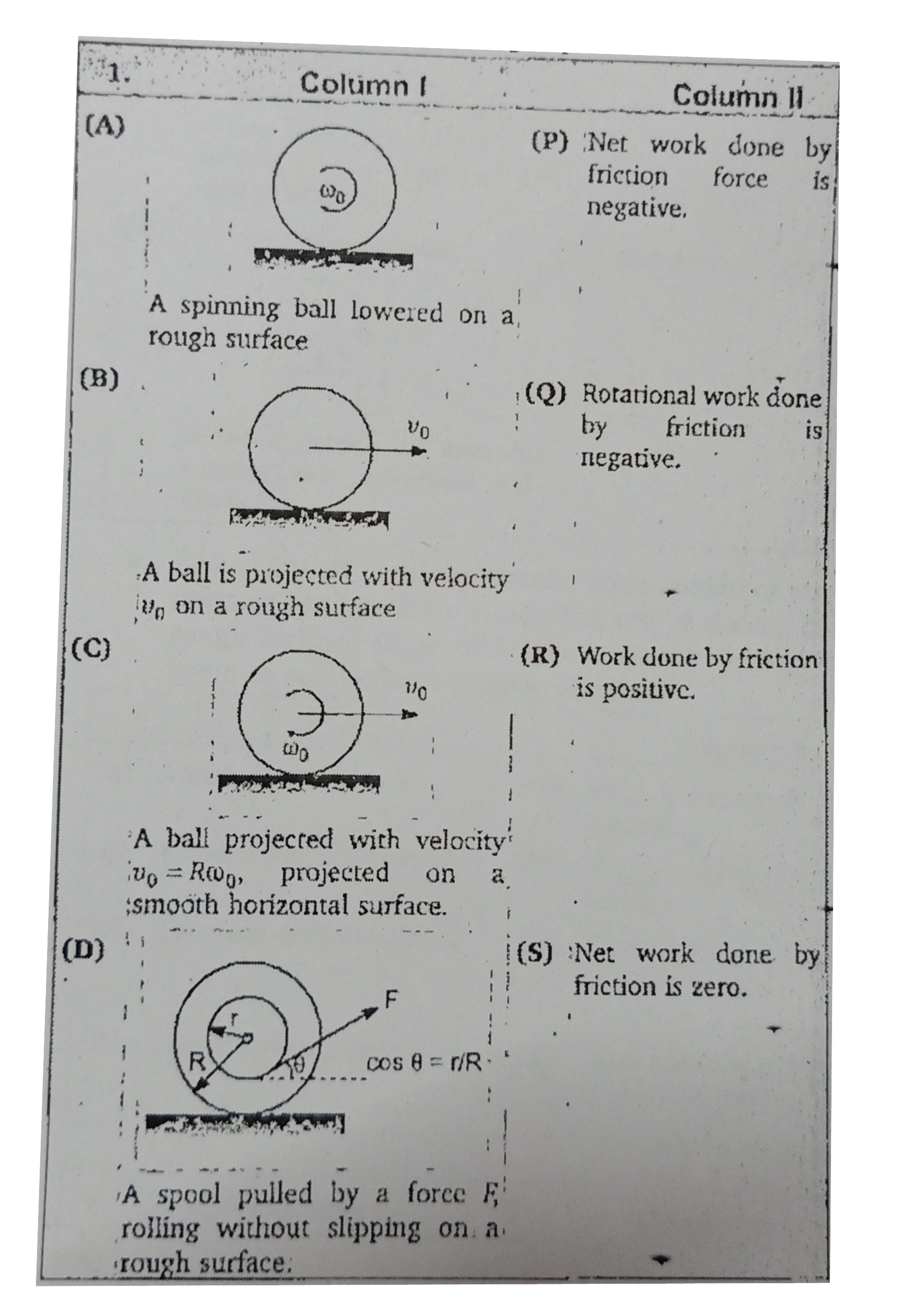
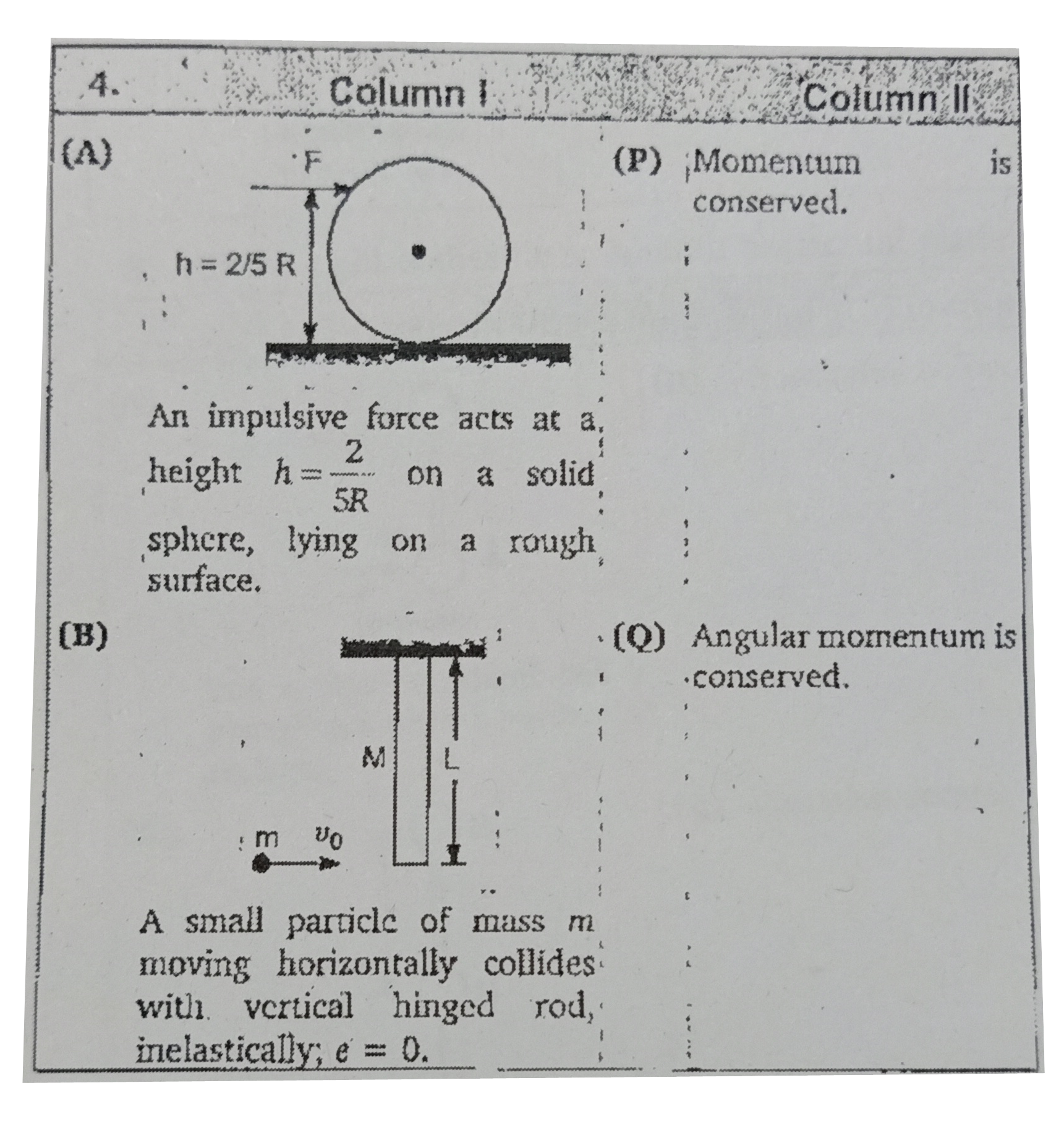
View SolutionMatch the following :
Column-I Column-II(A)Work done by all the forces(P)Change in potential energy(B)Work done by conservative forces(Q) Change in kinetic energy(C)Work done by external forces(R) Change in mechanical energy(S) None
View SolutionDuring an experiment, an ideal gas is found to obey a condition (p^2)/(rho) = "constant" . ( rho = density of the gas). The gas is initially at temperature (T), pressure (p) and density rho . The gas expands such that density changes to rho//2 .
View SolutionThe dimensions of γ in the relation v=√γpρ (where v is velocity, p is pressure , ρ is density)
View SolutionAn ideal gas expands following a relation P2ρ= constant, where P = pressure and ρ = density of the gas. The gas is initially at temperature T and density ρ and finally its density becomes ρ3.
(a) Find the final temperature of the gas. (b) Draw the P – T graph for the process.
View SolutionThe pressure and density of a gas (γ=1.5) changes for (P,ρ)→(P',ρ') during adiabatic changes if ρ'/ρ=32,thenP'/P will be
View SolutionIf P is the pressure of a gas and rho is its density , then find the dimension of velocity in terms of P and rho .
View SolutionIt P is the pressure and rho is the density of a gas, then P and rho are realted as :
View SolutionIf V=√γpρ, then the dimensions of γ will be (where p is pressure, ρ is density and V is velocity) :
View SolutionDuring an experiment, an ideal gas is found to obey a condition p2ρ=constant. (ρ = density of the gas). The gas is initially at temperature (T), pressure (p) and density ρ. The gas expands such that density changes to ρ/2.
View SolutionIf p is the pressure of a gas and ρ is its density, then dimension of velocity is given by ______ .
View Solutionदाब (p), परम ताप (T) एवं घनत्व (ρ) के पदों में किसी गैस का समीकरण है
View SolutionThe pressure and density of gas (γ=75) changes adiabatically from (P,ρ) to (P',ρ'). If ρ'ρ=32, then find the ratio of P'P :
View SolutionIf V=√γPρ,V =velocity, P= pressure ρ= density, then dimension of γ will be …….
View Solution

