In a set of experiment on a hydrogen on a hypotherical one-electron atom, the wavelength of the photons emitted from transition ending in the ground state (n=1) are shown in the energy level diagram
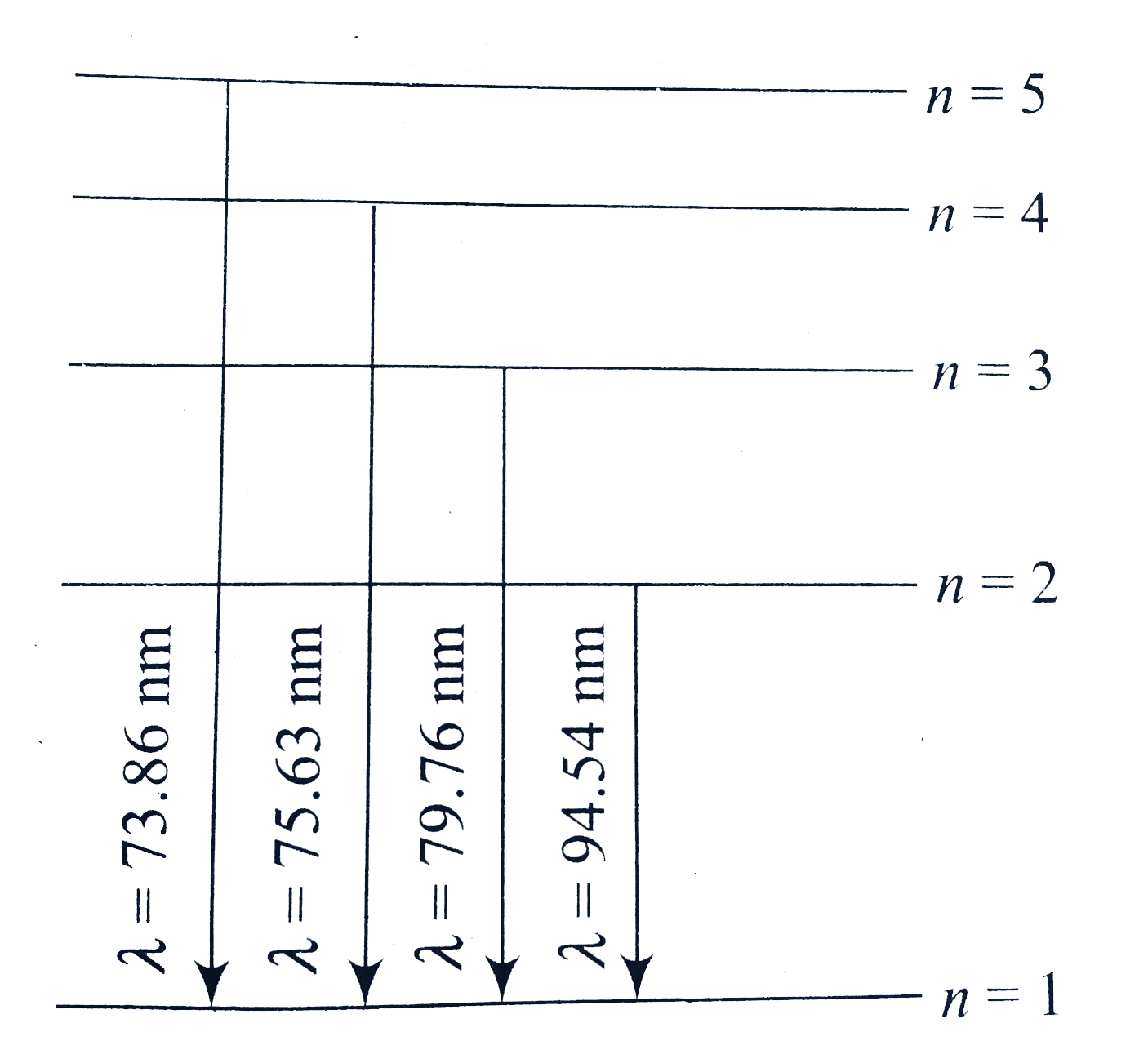
λ5→1=73.86nm
λ4→1=75.63nm
λ3→1=79.76nm
λ2→1=94.54nm
The possible energy of the atom in n=3 cannot be
−19.5eV
−0.4875eV
−0.121eV
−7.8eV
The correct Answer is:D
The possible energy of the atom in n=3.
E03=E3Z2
For Z=1,E03=−1.95eV
Z=2,E03=−0.4875eV
Z=3,E03=−0.2166eV
Z=4,E03=−0.121eV
Topper's Solved these Questions
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:Integer
Explore 4 Videos
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:Fill In The Blanks
Explore 8 Videos
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:Multiple Correct
Explore 13 Videos
- ALTERNATING CURRENTBook:CENGAGE PHYSICS ENGLISHChapter:ALTERNATING CURRENTExercise:Single Correct
Explore 10 Videos
- CAPACITOR AND CAPACITANCEBook:CENGAGE PHYSICS ENGLISHChapter:CAPACITOR AND CAPACITANCEExercise:Integer
Explore 5 Videos
Similar Questions
In a set of experiment on a hydrogen on a hypotherical one-electron atom, the wavelength of the photons emitted from transition ending in the ground state (n=1) are shown in the energy level diagram
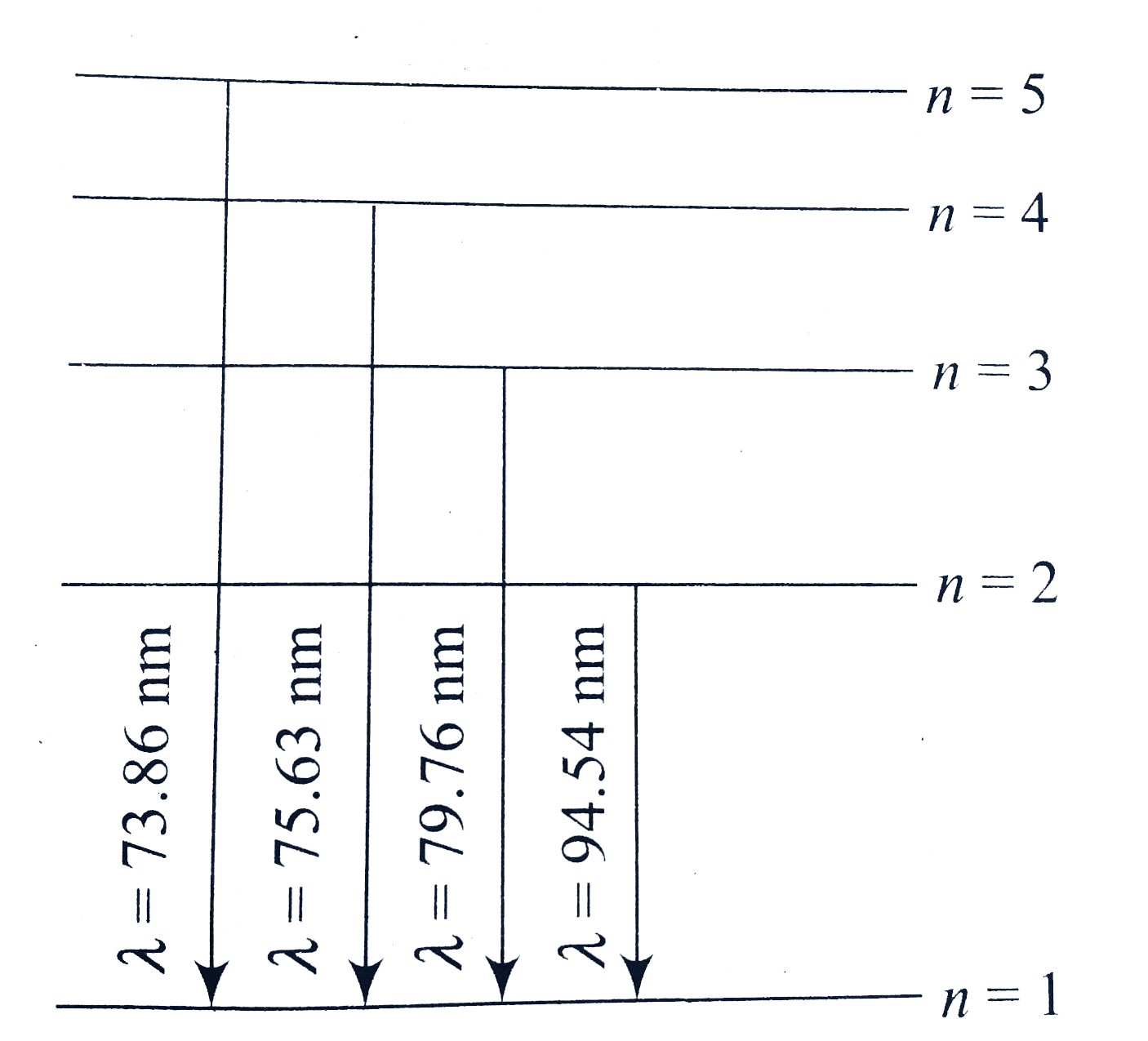
λ5→1=73.86nm
λ4→1=75.63nm
λ3→1=79.76nm
λ2→1=94.54nm
The energy of the atom in level n=1 is nearly
View SolutionIn a set of experiment on a hydrogen on a hypotherical one-electron atom, the wavelength of the photons emitted from transition ending in the ground state (n=1) are shown in the energy level diagram
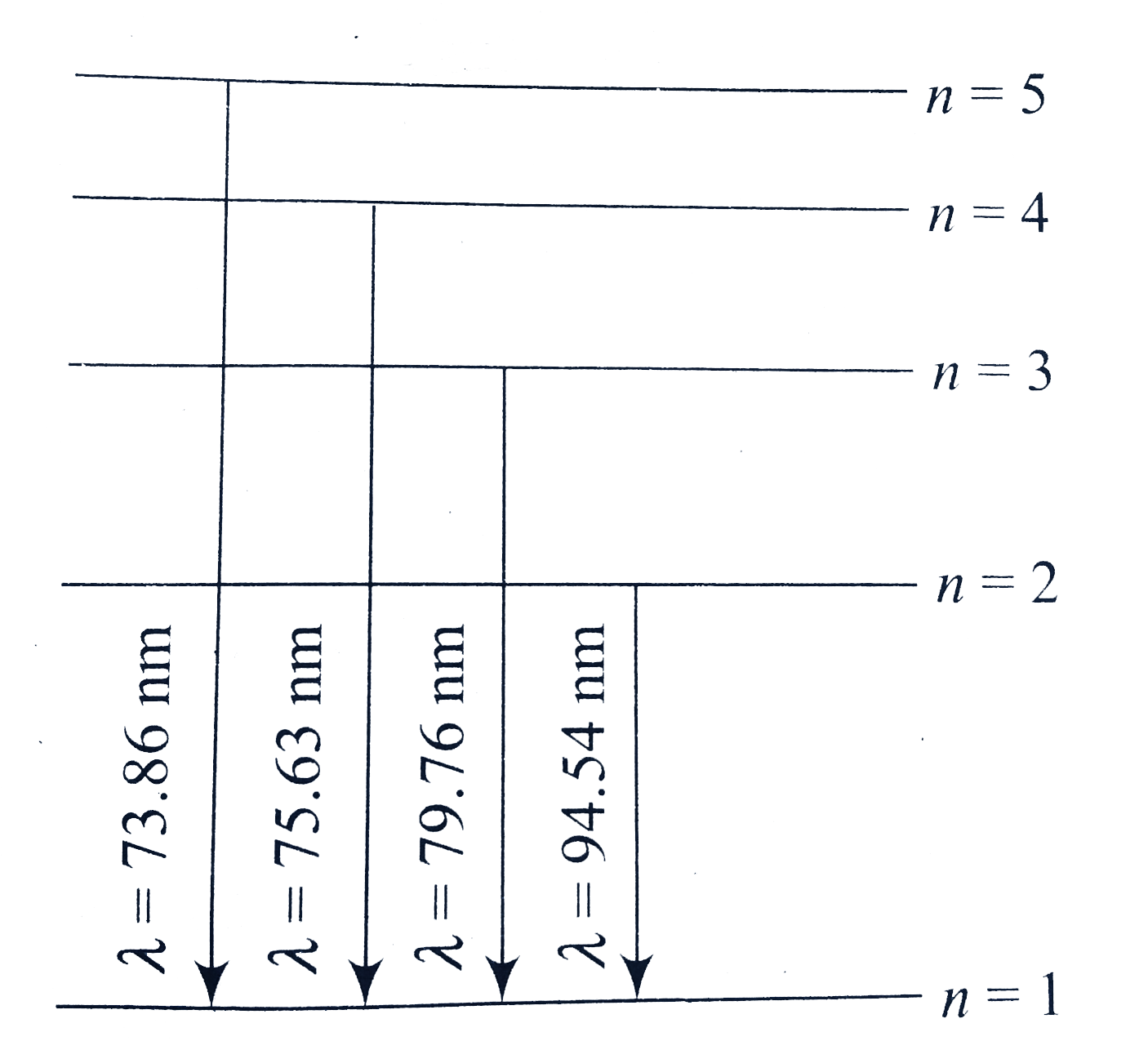
λ5→1=73.86nm
λ4→1=75.63nm
λ3→1=79.76nm
λ2→1=94.54nm
If an electron made a transition from n = 4 to n = 2 level, the wavelength of the light that it would emit is nearly
View SolutionThe energy levels of a hypotherical one electron atom are shown in figure
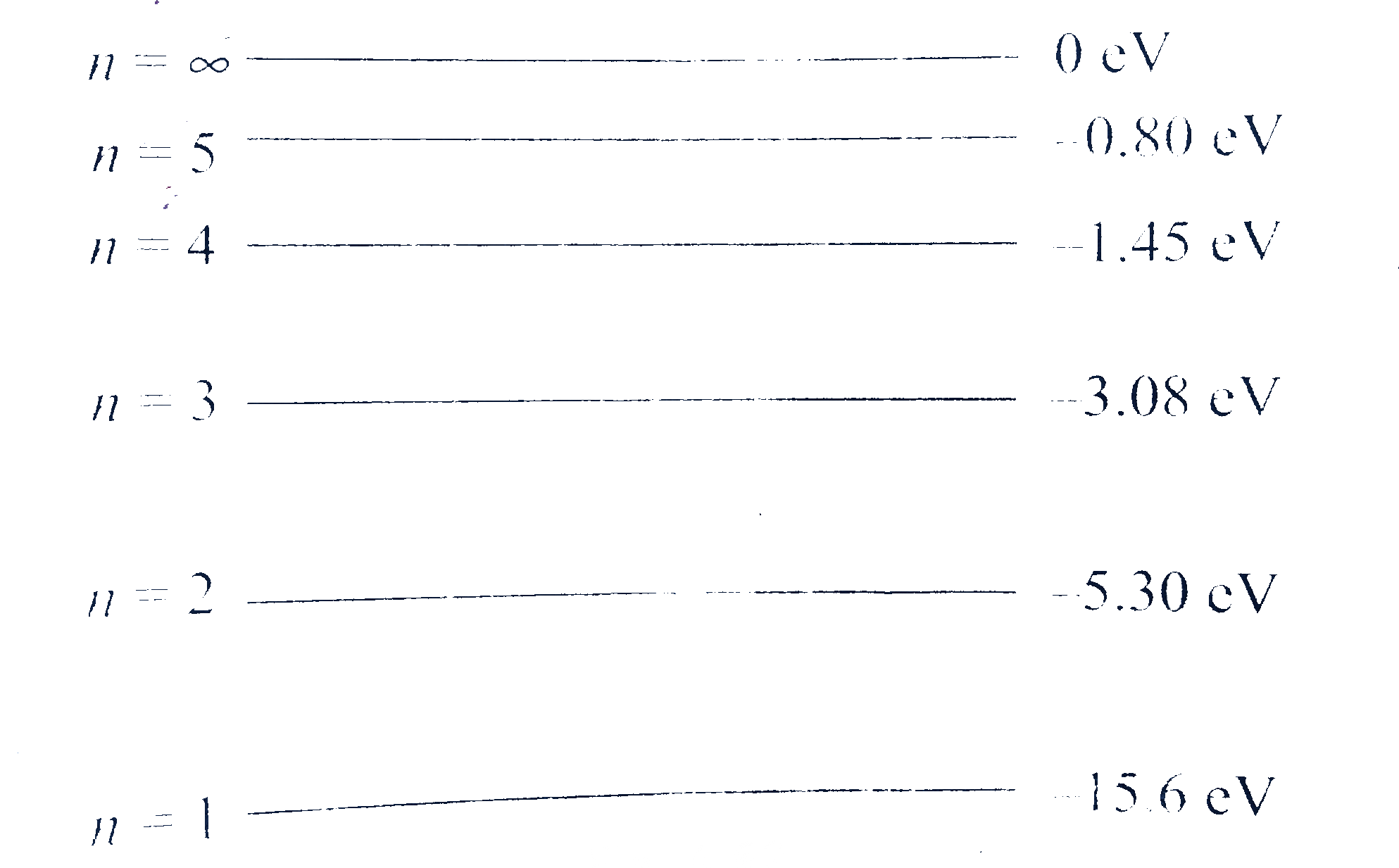
Find the wave number of the photon emitted for the transition n=3→n=1
View SolutionThe wavelengths of photons emitted by electron transition between two similar leveis in H and He+ are λ1 and λ2 respectively. Then :-
View SolutionThe wavelengths of photons emitted by electron transition between two similar leveis in H and He+ are λ1 and λ2 respectively. Then :-
View SolutionWhat is the wavelength of light emitted when the electron in a hydrogen atom undergoes transition from the energy level with n=4 to the energy level with n=1 ?
View SolutionThe energy of a photon of wavelength λ is
View SolutionFind the velocity of photoelectrons liberated by electromagnetic radiation of wavelength λ=18.0nm from stationary He+ ions in the ground state.
View SolutionThe electron in a hydrogen atom at rest makes a transition from n=2 energy state to the n=1 ground state.
find the energy (eV) of the emitted photon.
View SolutionIf lambda is the wavelength of hydrogen atom from the transition n=3→n=1,then what is the wavelength for doubly ionised lithium ion for same transition?
View Solution
