In producing chrlorine through electorlysis, 100 kW power at 125 V is being consumed. How much chlorine per minute is liberated ? Electrochemical equivalent for chlorine =0.367×10−6kgC−1
1.76×10−3kg
9.67×10−3kg
17.6×10−3kg
3.67×10−3kg
The correct Answer is:C
m=Z≤Z(PV)t
=(0.367×10−6)(100×103125)(60)
=17.61×10−3kg
Topper's Solved these Questions
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:MCQ
Explore 78 Videos
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:MCQs
Explore 613 Videos
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:MCQs
Explore 613 Videos
- MOTION IN TWO AND THREE DIMENSIONBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:MOTION IN TWO AND THREE DIMENSIONExercise:All Questions
Explore 47 Videos
- NEET 2020Book:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEET 2020Exercise:All Questions
Explore 45 Videos
Similar Questions
The damping force on an oscillator is directly proportional to the velocity. The units of the constant to proportionality are
View SolutionTwo masses as shown are suspended from a massless pulley. Calculate the acceleration of the system when masses aere left free:
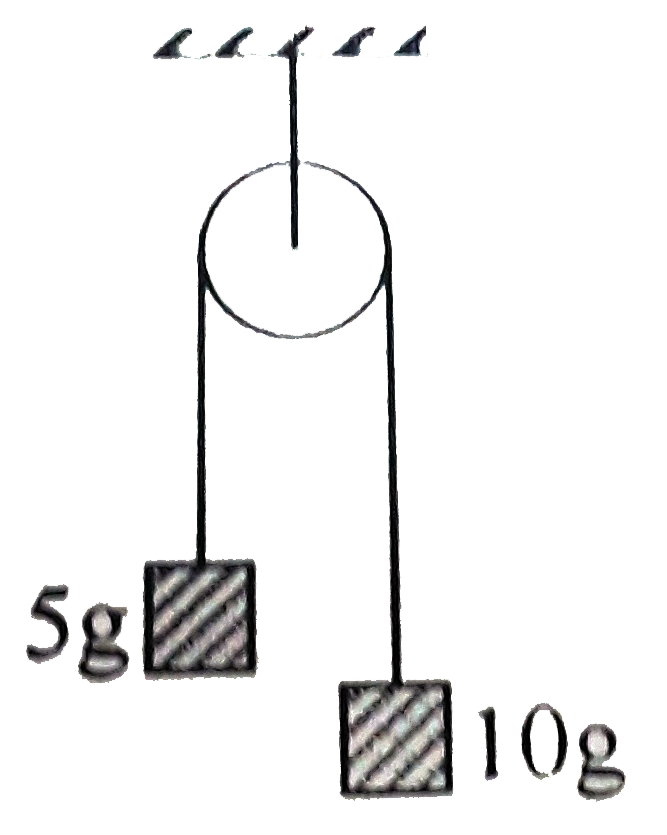
View SolutionTwo masses as shown are suspended from a massless pulley. Calculate the acceleration of the system when masses aere left free:
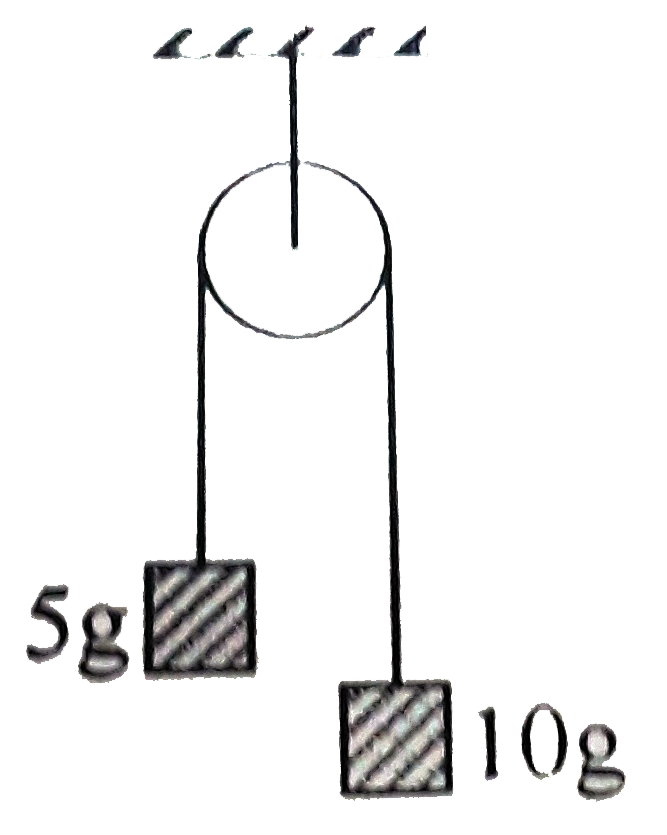
View SolutionThe x and y coordinates of the particle at any time are x=5t−2t2andy=10t respectively, where x and y are in meters and t in seconds. The acceleration of the particle at t=2s is:
View SolutionLight with an average flux of 20Wcm2 falls on a non-reflecting surface at normal incidence having surface area 20cm2. The energy received by the surface during time span of 1 minute is:
View SolutionIn producing chlorine through electrolysis 100 W power at 125 V is being consumed. How much chlorine per min is liberated ? ECE of chlorine is 0.367 xx 10^(6) kg//C
View SolutionIn producing chrlorine through electorlysis, 100 kW power at 125 V is being consumed. How much chlorine per minute is liberated ? Electrochemical equivalent for chlorine =0.367×10−6kgC−1
View SolutionHow many grams of chorine can be produced by the eletrolysis of molten NaCl with a currect of a A or 15 min utes .ECE of chlorine =0.367×10−7kgC−1
View Solution1किलोवाट के विधुत बल्ब में 1 मिनट में कितनी ऊर्जा व्यय होगी ?
View SolutionIn producing chrlorine through electorlysis, 100 kW power at 125 V is being consumed. How much chlorine per minute is liberated ? Electrochemical equivalent for chlorine =0.367×10−6kgC−1
View SolutionHow much chlorine will be liberated on passing one ampere current for 30 minutes through NaCl solution ?
View Solutionविद्युत अपघटन प्रक्रम द्वारा क्लोरीन बनाने में 125 वोल्ट पर 100kW शक्ति की खपत हो रही है। प्रति मिनट कितनी क्लोरीन विमुक्त हो रही है, क्लोरीन के लिए विद्युत रासायनिक तुल्यांक 0.367×10−6 किग्रा/कूलॉम है-
View SolutionHow much chlorine will be liberated on passing one ampere current for 30 minutes through aqueous NaCl solution?
View SolutionChlorine output from electrical decomposition consumes 100 kW power at 125 V. How much chlorine is free per minute? Of chlorine ece = 0 . 367 xx 10 ^(-6) kg //C is.
View SolutionIn producing chlorine by electrolysis 100 kw power at 125 V is consumed. How much chlorine per minute is liberated.
View SolutionNaCl विलयन में 30 मिनट तक 1 ऐम्पियर धारा को प्रवाहित करने पर कितनी क्लोरीन मुक्त होगी?
View Solution
