In the spectrum of singly ionized helium , the wavelength of a line abserved is almost the same as the first line of Balmer series of hydrogen . It is due to transition of electron
omn1=6→n2=4
omn5=6→n2=3
omn1=4→n2=2
omn1=3→n2=2
The correct Answer is:A
For the first line of Balmer series of hydrogen
1λ=R(122−132)=5R36⇒λ=365R
For singly ionized helium (Z=2),
1λ'=4R(1n21−1n22)
Given λ'=λ
For n1=6→n2=4
1λ'=4R(142−162)=20R144=5R36
It corresponds to transition from n1=6→n2=4.
Topper's Solved these Questions
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:Multiple Correct
Explore 13 Videos
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:Linked Comprehension
Explore 62 Videos
- ATOMIC PHYSICSBook:CENGAGE PHYSICS ENGLISHChapter:ATOMIC PHYSICSExercise:Subject
Explore 17 Videos
- ALTERNATING CURRENTBook:CENGAGE PHYSICS ENGLISHChapter:ALTERNATING CURRENTExercise:Single Correct
Explore 10 Videos
- CAPACITOR AND CAPACITANCEBook:CENGAGE PHYSICS ENGLISHChapter:CAPACITOR AND CAPACITANCEExercise:Integer
Explore 5 Videos
Similar Questions
Which of the following statements are true?
View SolutionHydrogen is the simplest atom of nature. There is one proton in its nucleus and an electron moves around the nucleus in a circular orbit. According to Niels Bohr's, this electron moves in a stationary orbit, if emits no electromagnetic radiation. The angular momentum of the electron is quantized , i.e., mvr=(nh/2π), where m= mass of the electron , v=velocity of the electron in the orbit , r=radius of the orbit , and n=1,2,3.... When transition takes place from Kth orbit to Jth orbit, energy photon is emitted. If the wavelength of the emitted photon is λ.
we find that 1λ=R[1J2−1K2], where R is
Rydberg's constant.
On a different planed, the hydrogen atom's structure was somewhat different from ours. The angular momentum of electron was P=2n(h/2π). i.e., an even multipal of (h/2π).
Answer the following questions regarding the other planet based on above passage:
In our world, the velocity of electron is v0 when the hydrogen atom is in the ground state on the other planet should be
View SolutionThe peak emission from a black body at a certain temprature occurs at a wavelength of 9000Å. On increase its temperature , the total radiation emmited is increased its 81 times. At the intial temperature when the peak radiation from the black body is incident on a metal surface , it does not cause any photoemission from the surface . After the increase of temperature, the peak from the black body caused photoemission. To bring these photoelectrons to rest , a potential equivalent to the excitation energy between n=2andn=3 bohr levels of hydrogen atoms is required. Find the work function of the metal.
View SolutionThe rms current in an AC circuit is 2A. If the wattless current be √3A , what is the power factor of the circuit?
View SolutionEach capacitance shown in is in μF. Find the charge on 6μF.
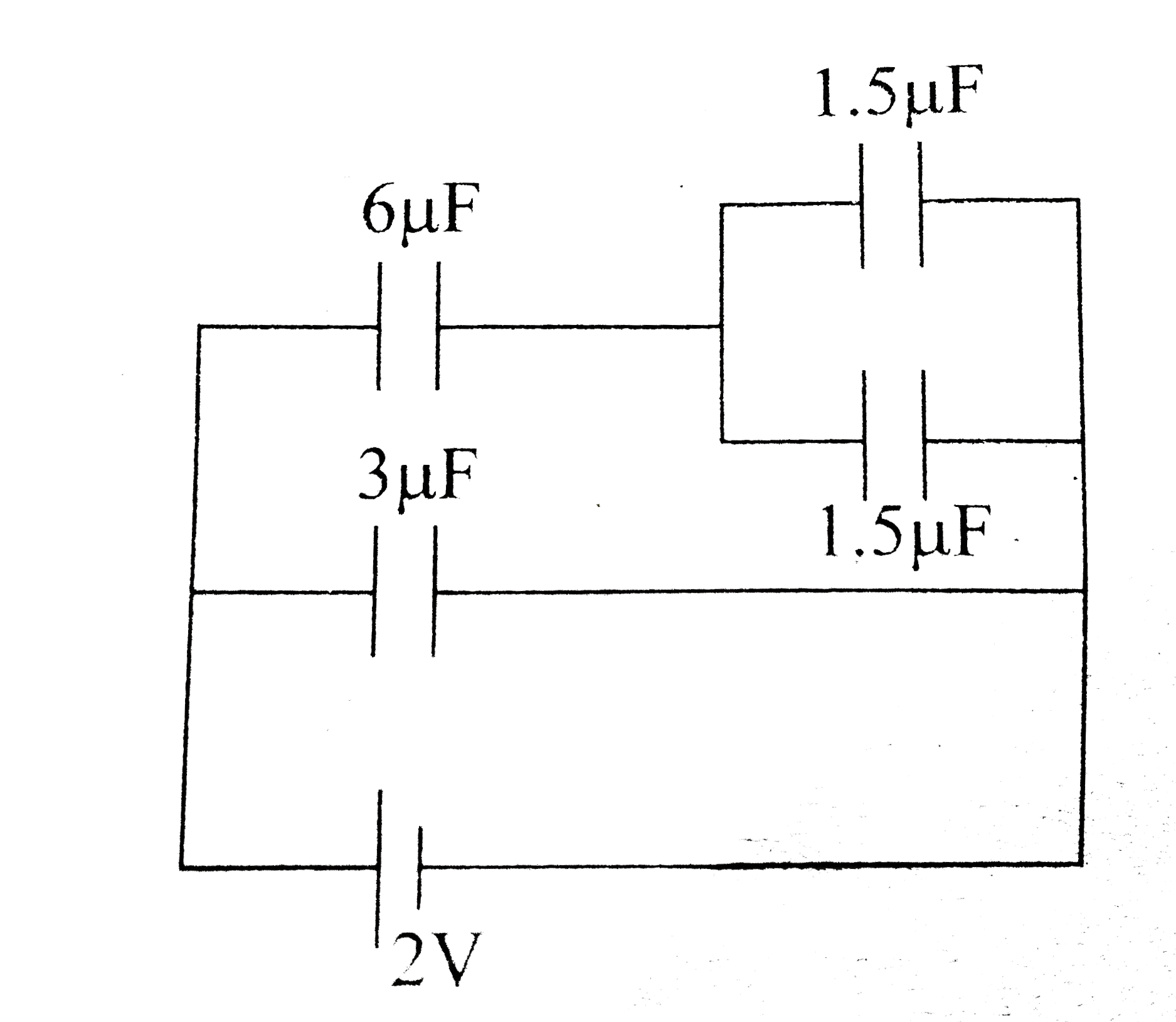 .
.
View SolutionOne of the lines in the emission spectrum of Li2+ has the same wavelength as that of the second line of Balmer series in hydrogen spectrum. The electronic transition correspnding to this line is.
View SolutionIn the spectrum of singly ionized helium , the wavelength of a line abserved is almost the same as the first line of Balmer series of hydrogen . It is due to transition of electron
View SolutionIn the spectrum of singly ionized helium , the wavelength of a line abserved is almost the same as the first line of Balmer series of hydrogen . It is due to transition of electron from n_(1) = 6 to n_(2) = ^(''**'') . What is the value of (''**'') .
View SolutionOne of the lines in the emission spectrum of Li2+ has the same wavelength as that of the 2nd line of Balmer series in hydrogen spectrum. The electronic transition corresponding to this line is:
View Solutionहाइड्रोजन परमाणु की बामर श्रेणी की प्रथम स्पेक्ट्रमी रेखा की तरंगदैर्घ्य 6561Å है। एकल-आयनित हीलियम परमाणु की बामर श्रेणी की द्वितीय रेखा की तरंगदैर्घ्य होगी :
View Solutionहाइड्रोजन परमाणु के बामर श्रेणी में प्रथम स्पेक्ट्रमी रेखा की तरंगदैर्घ्य 6561Å हैं , तब एकल आयनित हीलियम परमाणु के बामर श्रेणी की व्दितीय स्पेक्ट्रमी की तरंगदैर्घ्य होगी -
View SolutionThe wavelength of the first spectral line in the Balmer series of hydrogen atom is 6561 Å. The wavelength of the second spectral line in the Balmer series of singly ionized helium atom is
View SolutionThe wavelength of the first spectral line in the Balmer series of hydrogen atom is 6561 Å. The wavelength of the second spectral line in the Balmer series of singly- ionized helium atom is :
View SolutionThe wavelength of the first spectral line in the Balmer series of hydrogen atom is 6561 Å. If the wavelength of the spectral line in the Balmer series of singly-ionized helium atom is 1215 when electron jumps from n2 to n1, then n2 and n1, are
View SolutionIn hydrogen spectrum Lα line arises due to transition of electron from the orbit n=3 to the orbit n=2. In the spectrum of singly ionized helium there is a line having the same wavelength as that of the Lα line. This is due to the transition of electron from the orbit:
View Solution
