In which of the following transition will the wavelength be minimum ?
n=5 to n=4
n=4 to n=3
n=3 to n=2
n=2 to n=1
The correct Answer is:D
To determine which transition results in the minimum wavelength, we can use the Rydberg formula for hydrogen-like atoms, which is given by:
1λ=RZ2(1n21−1n22)
Where:
- λ is the wavelength,
- R is the Rydberg constant,
- Z is the atomic number,
- n1 and n2 are the principal quantum numbers of the two energy levels involved in the transition, with n2>n1.
1. Identify the transitions and their quantum numbers:
- Option A: n1=2, n2=5
- Option B: n1=3, n2=4
- Option C: n1=2, n2=3
- Option D: n1=1, n2=2
2. Calculate the wavelength for each transition:
- Option A: Transition from n=5 to n=2
1λA=R(122−152)=R(14−125)=R(25−4100)=R(21100)
λA=10021R
- Option B: Transition from n=4 to n=3
1λB=R(132−142)=R(19−116)=R(16−9144)=R(7144)
λB=1447R
- Option C: Transition from n=3 to n=2
1λC=R(122−132)=R(14−19)=R(9−436)=R(536)
λC=365R
- Option D: Transition from n=2 to n=1
1λD=R(112−122)=R(1−14)=R(34)
λD=43R
3. Compare the wavelengths:
- λA=10021R
- λB=1447R
- λC=365R
- λD=43R
4. Determine the minimum wavelength:
- To find the minimum wavelength, we need to compare the values obtained:
- λA≈4.76
- λB≈20.57
- λC≈7.2
- λD≈1.33
From the calculations, we find that the minimum wavelength occurs for Option D: Transition from n=2 to n=1.
Final Answer:
The transition with the minimum wavelength is from n=2 to n=1.
- λ is the wavelength,
- R is the Rydberg constant,
- Z is the atomic number,
- n1 and n2 are the principal quantum numbers of the two energy levels involved in the transition, with n2>n1.
- Option A: n1=2, n2=5
- Option B: n1=3, n2=4
- Option C: n1=2, n2=3
- Option D: n1=1, n2=2
- Option A: Transition from n=5 to n=2
1λA=R(122−152)=R(14−125)=R(25−4100)=R(21100)
λA=10021R
1λB=R(132−142)=R(19−116)=R(16−9144)=R(7144)
λB=1447R
1λC=R(122−132)=R(14−19)=R(9−436)=R(536)
λC=365R
1λD=R(112−122)=R(1−14)=R(34)
λD=43R
- λA=10021R
- λB=1447R
- λC=365R
- λD=43R
- To find the minimum wavelength, we need to compare the values obtained:
- λA≈4.76
- λB≈20.57
- λC≈7.2
- λD≈1.33
The transition with the minimum wavelength is from n=2 to n=1.
Topper's Solved these Questions
- ATOMSBOOK - DC PANDEY ENGLISHCHAPTER - ATOMSEXERCISE - Check point 12.3
15 Videos
- ATOMSBOOK - DC PANDEY ENGLISHCHAPTER - ATOMSEXERCISE - Taking it together
117 Videos
- ATOMSBOOK - DC PANDEY ENGLISHCHAPTER - ATOMSEXERCISE - Check point 12.1
14 Videos
- ALTERNATING CURRENTBOOK - DC PANDEY ENGLISHCHAPTER - ALTERNATING CURRENTEXERCISE - JEE MAIN
62 Videos
- CAPACITORSBOOK - DC PANDEY ENGLISHCHAPTER - CAPACITORSEXERCISE - OBJECTIVE_TYPE
1 Video
Similar Questions
X-rays were discovered by
View SolutionIncrease in which of the following increases the penetrating power of the X-rays ?
View SolutionThe radius of the atom is of the order of
View SolutionIn the given arrangement, the loop is moved with consisant velocity in uniform magnetic field B in a restricted a region of width a. The time for which the emf is induced in the cirucit is
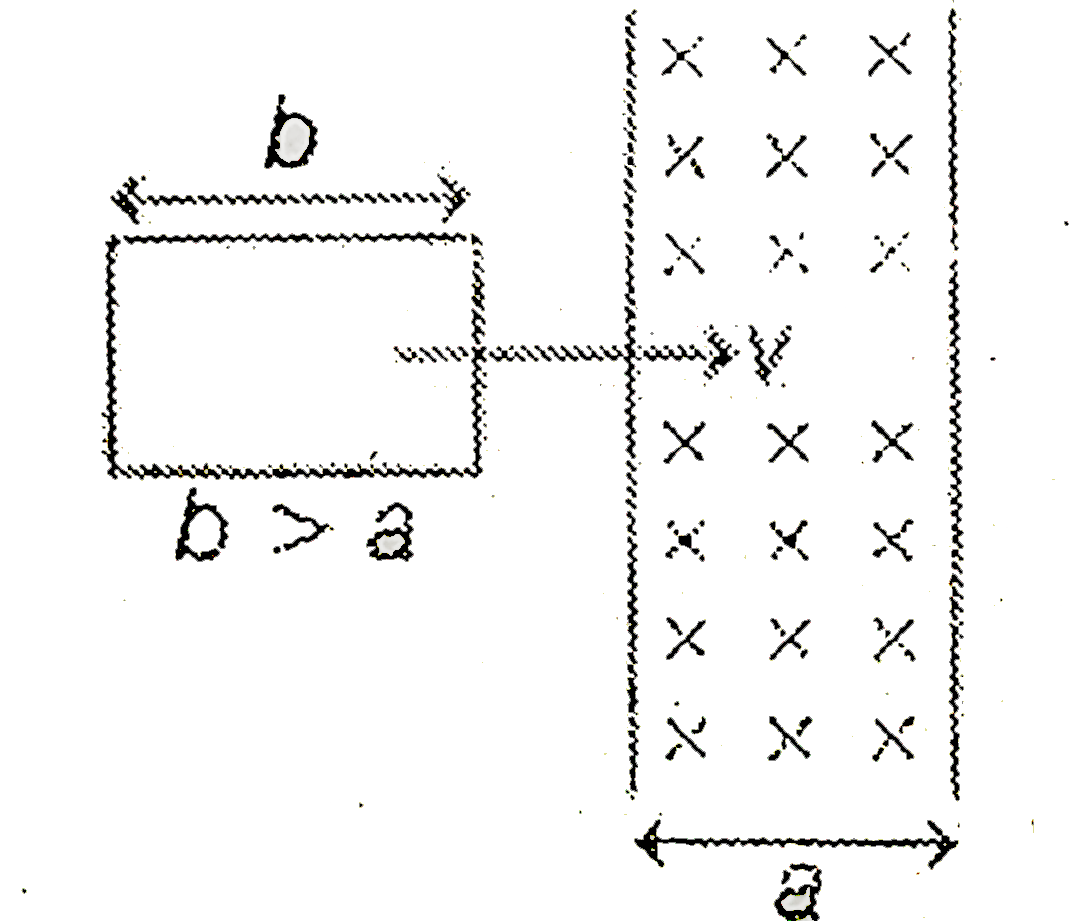
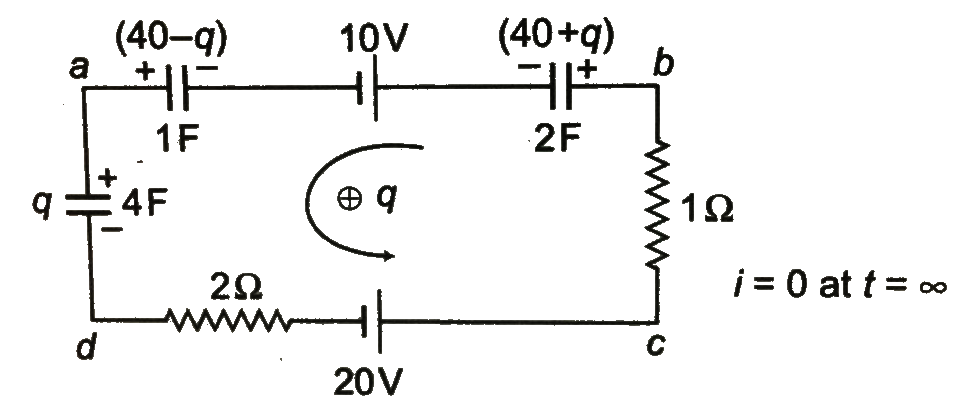
View SolutionIn the circuit shown as potential difference of 60V is appliled across AB. The potential difference between the points M and N is
View SolutionIn which of the following transition, the wavelength will be minimum :
View SolutionWhich of the following transitions have minimum wavelength
View SolutionWhich of the following transitions have minimum wavelength
View SolutionThe series corresponding to minimum wavelength transition in H-atom :-
View SolutionThe wavelength will be minimum for which of the following electronic transition in an unielectron species?
View SolutionThe transition of electron in hydrongen atom having minimum wavelength is :
View Solutionहाइड्रोजन परमाणु के लिए , निम्न में से किस संक्रमण में उत्सर्जित फोटॉन का तरंगदैर्ध्य न्यूनतम होगा
View SolutionWhich of the following transitions will have minimum wavelength ?
View SolutionH-परमाणु में न्यूनतम तरंगदैर्ध्य संक्रमण किस श्रेणी के अंतर्गत होते है -
View Solutionनिम्न में से कौन से संक्रमण में तरंग दैर्ध्य न्यूनतम होगी?
View SolutionWhich of the following transitions will have minimum wavelength ?
View Solution

