Mn and Mp represet the mass of neutron and proton respectively. An element having mass M has N neutron and Z-protons, then the correct relation will be : -
M<[NMn+ZMP]
M>[NMn+ZMP]
M=[NMn+ZMP]
M=N[Mn+MP]
The correct Answer is:A
Step by step video solution for M_n and M_p represet the mass of neutron and proton respectively. An element having mass M has N neutron and Z-protons, then the correct relation will be : - by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- NUCLEAR PHYSICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - NUCLEAR PHYSICSEXERCISE - ddp.5.2
15 Videos
- NUCLEAR PHYSICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - NUCLEAR PHYSICSEXERCISE - ddp.5.3
15 Videos
- NUCLEAR PHYSICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - NUCLEAR PHYSICSEXERCISE - Integer type
3 Videos
- MISCELLANEOUS VOLUME 5BOOK - CENGAGE PHYSICS ENGLISHCHAPTER - MISCELLANEOUS VOLUME 5EXERCISE - Integer
11 Videos
- PHOTOELECTRIC EFFECTBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - PHOTOELECTRIC EFFECTEXERCISE - Integer Type
4 Videos
Similar Questions
The mass defect in a particular nuclear reaction is 0.3 grams. The amont of energy liberated in kilowatt hours is.
(Velocity of light =3×108m/s).
View SolutionIn the disintegration series
.23892U→αX−−→β−.AZY the values of Z and A, respectively, will be
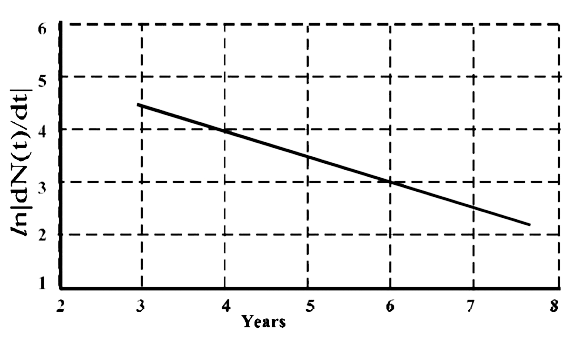
View SolutionTo determine the half life of a radioactive element , a student plote a graph of in ∣∣∣dN(t)dt∣∣∣versust,Here∣∣∣dN(t)dt∣∣∣ is the rate of radioatuion decay at time t , if the number of radoactive nuclei of this element decreases by a factor of p after 4.16 year the value of p is
View SolutionA charged particle enters a uniform magnetic field with velocity v0=4m/s perpendicular to it, the length of magnetic field is x=(√32)R, where R is the radius of the circular path of the particle in the field. Find the magnitude of charge in velocity (in m/s) of the particle when it comes out of the field.
View SolutionAn α particle and a proton are accelerated from rest by a potential difference of 100V. After this, their de-Broglie wavelengths are λa
and λp respectively. The ratio λpλa, to the nearest integer, is.
View SolutionIf m,mn and mp are masses of .ZXA nucleus, neutron and proton respectively.
View SolutionMn and Mp represent mass of neutron and proton respectively. If an element having atomic mass M has N−neutron and Z-proton, then the correct relation will be :
View SolutionM_p denotes the mass of a proton and M_n that of a neutron. A given nucleus, of binding energy B , contains Z protons and N neutrons. The mass M(N,Z) of the nucleus is given by.
View SolutionmP and mn are masses of proton and neutron respectively. An element of mass m has Z protons and N neutrons, then
View Solutionm_(p) and m_(n) are masses of proton and neutron respectively. An element of mass M has Z protons and N neutrons then
View SolutionA nucleus containing Z protons and N neutrons has a mass M . If the mass of a proton is mp and that of a neutron is mn , then the mass defect of the nucleus is
View SolutionM_p Is the mass of the proton M_n Is the mass of the neutron. If the bond of a nucleus with Z protons and N neutrons is B then the mass of that nucleus is M (N, Z), (where c is the speed of light)
View SolutionMn तथा Mp क्रमशः न्यूट्रॉन तथा प्रोटॉन के द्रव्यमानों को प्रदर्शित करते हैं | यदि किसी तत्व का परमाणु द्रव्यमान M तथा इसमें N न्यूट्रॉन व Z - प्रोटॉन हो तो सही सम्बन्ध होगा
View SolutionM_(n) and M_(p) represent mass of neutron and proton respectively. If an element having nuclear mass M has N neutron and Z protons, then the correct relation will be
View Solutionयदि Mn व Mp क्रमश: न्यूट्रॉन व प्रोट्रोन के द्रव्यमान है तो एक तत्व जिसका नाभिकीय द्रव्यमान M है N न्यूट्रान व Z प्रोट्रॉन है, तो सही सम्बन्ध है :-
View SolutionMp एक प्रोटॉन का द्रव्यमान व्यक्त करता है और Mn एक न्यूट्रॉन का एक नाभिक विशेष में Z प्रोटॉन और N न्यूटरॉन हैं और इसकी आबंधन ऊर्जा B है। M (N, Z) व्यक्त होगा (c प्रकाश का वेग है)
View Solution

