One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in (figure). The change in internal energy of the gas during the transition is (γ=3/5)
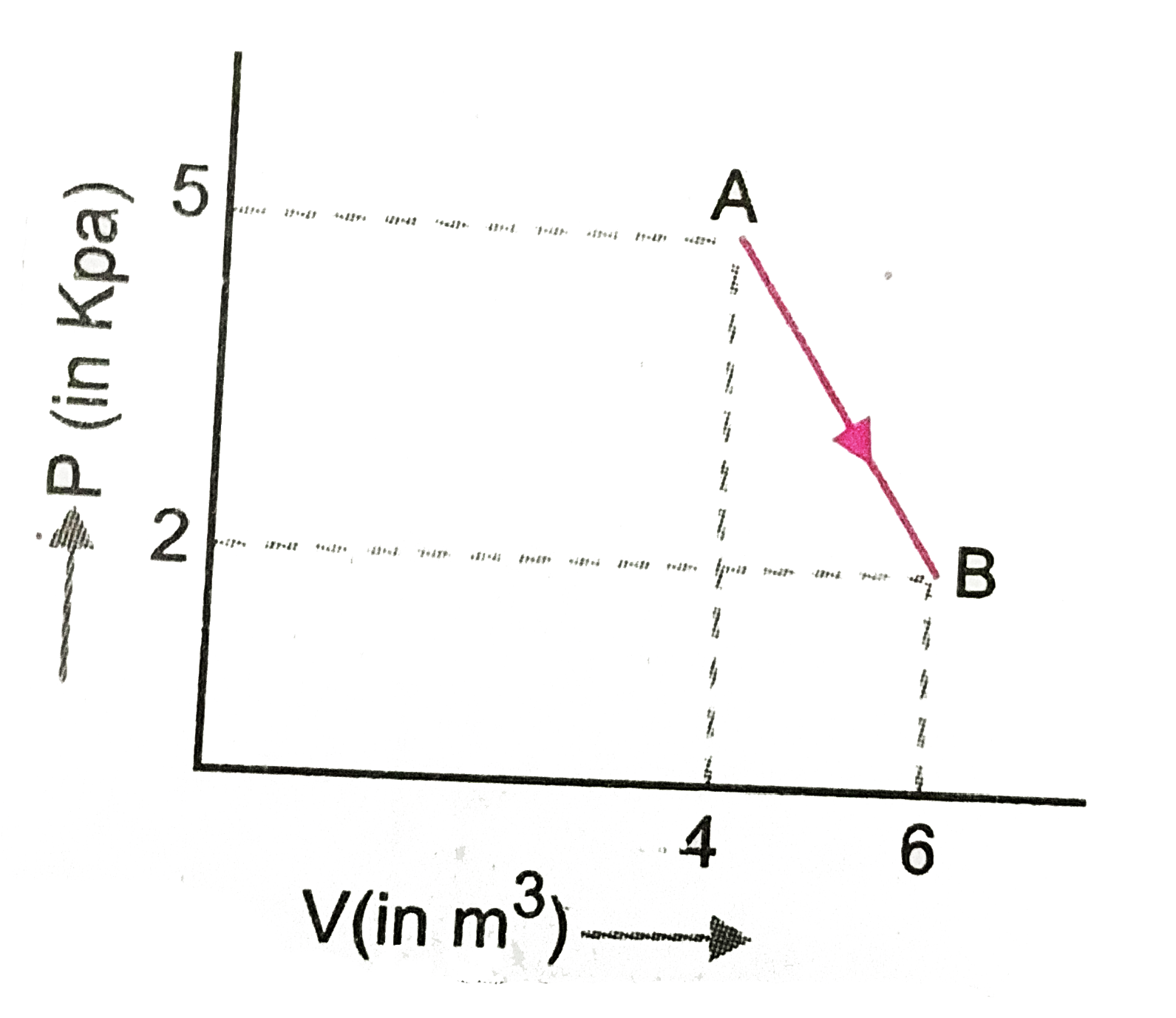
20 KJ
−12kJ
−20kJ
20 J
The correct Answer is:C
Step by step video, text & image solution for One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in (figure). The change in internal energy of the gas during the transition is (gamma=3//5) by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure. The change in internal energy of the gas during the transition is
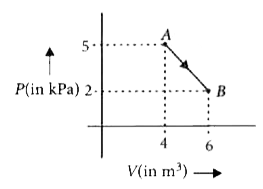
View SolutionOne mole of an ideal diatomic gas undergoes a transition from A and B along a path AB as shown in the figure The change in internal energy of the gas during the transition is
View SolutionOne mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in (figure). The change in internal energy of the gas during the transition is (gamma=3//5)
View Solutionएक आदर्श द्विपरमानुक गैस के 1 मोल को , AB पथ के अनुदिश A से B तक ले जाता है।
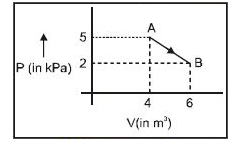
इस प्रक्रम के दौरान गैस की आंतरिक ऊर्जा में परिवर्तन होगा:
View SolutionOne mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in (figure). The change in internal energy of the gas during the transition is (γ=3/5)

View SolutionOne mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure
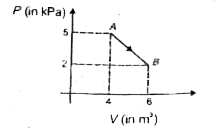
The change to internal energy of the gas during the transition is
View Solution0.5 moles of an ideal monoatomic gas undergoes a thermodynamic process from A to B as shown in figure. The change in internal energy of the gas during the process from A to B is
View SolutionOne mole of an ideal monoatomic gas is caused to go through the cycle shown in figure. Then the change in the internal energy of gas from a to b and b to c is respectively
View Solution
