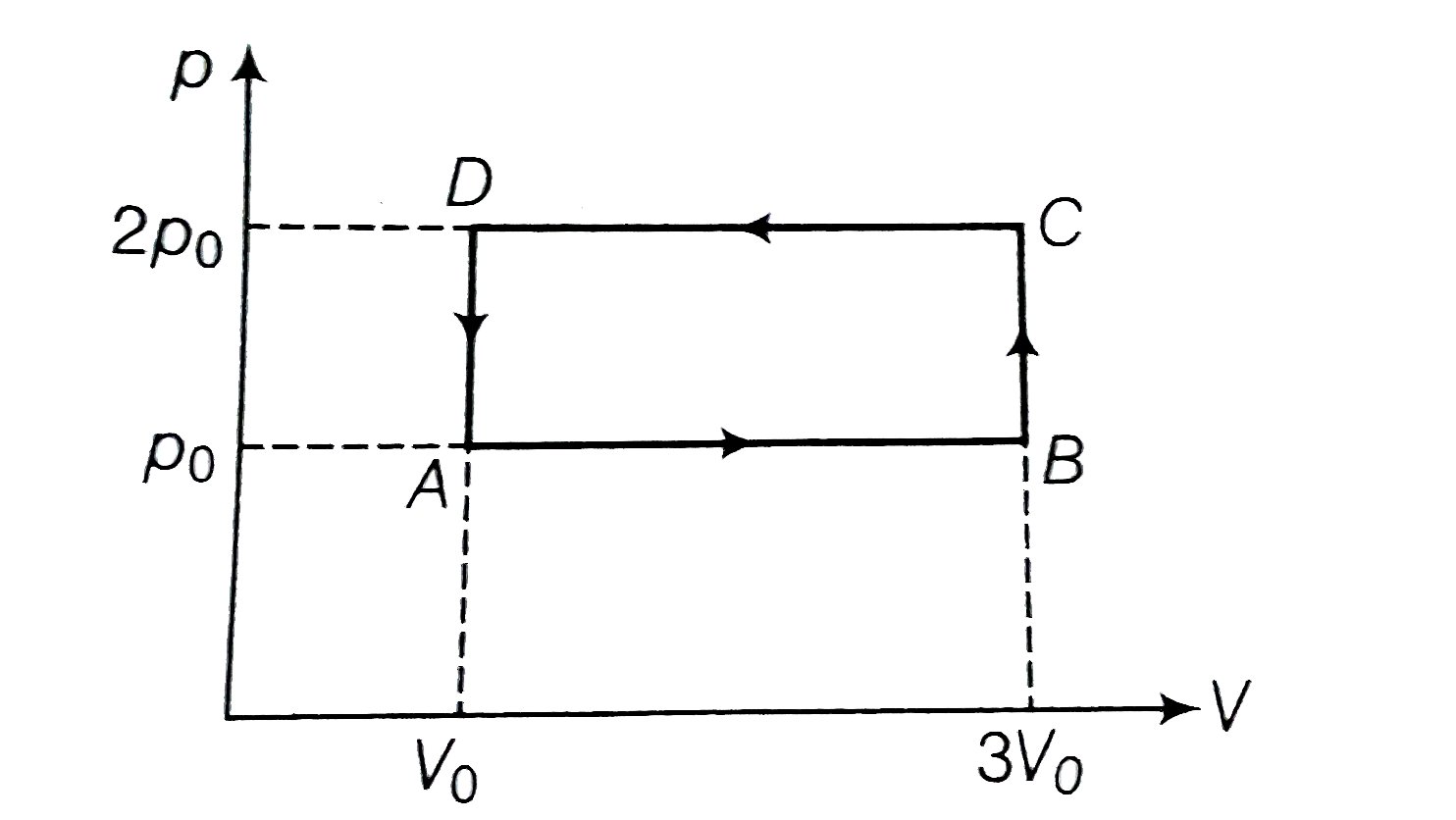
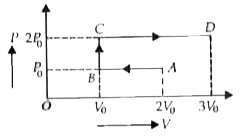
P−V diagram of an ideal gas is as shown in figure. Work done by the gas in process ABCD is
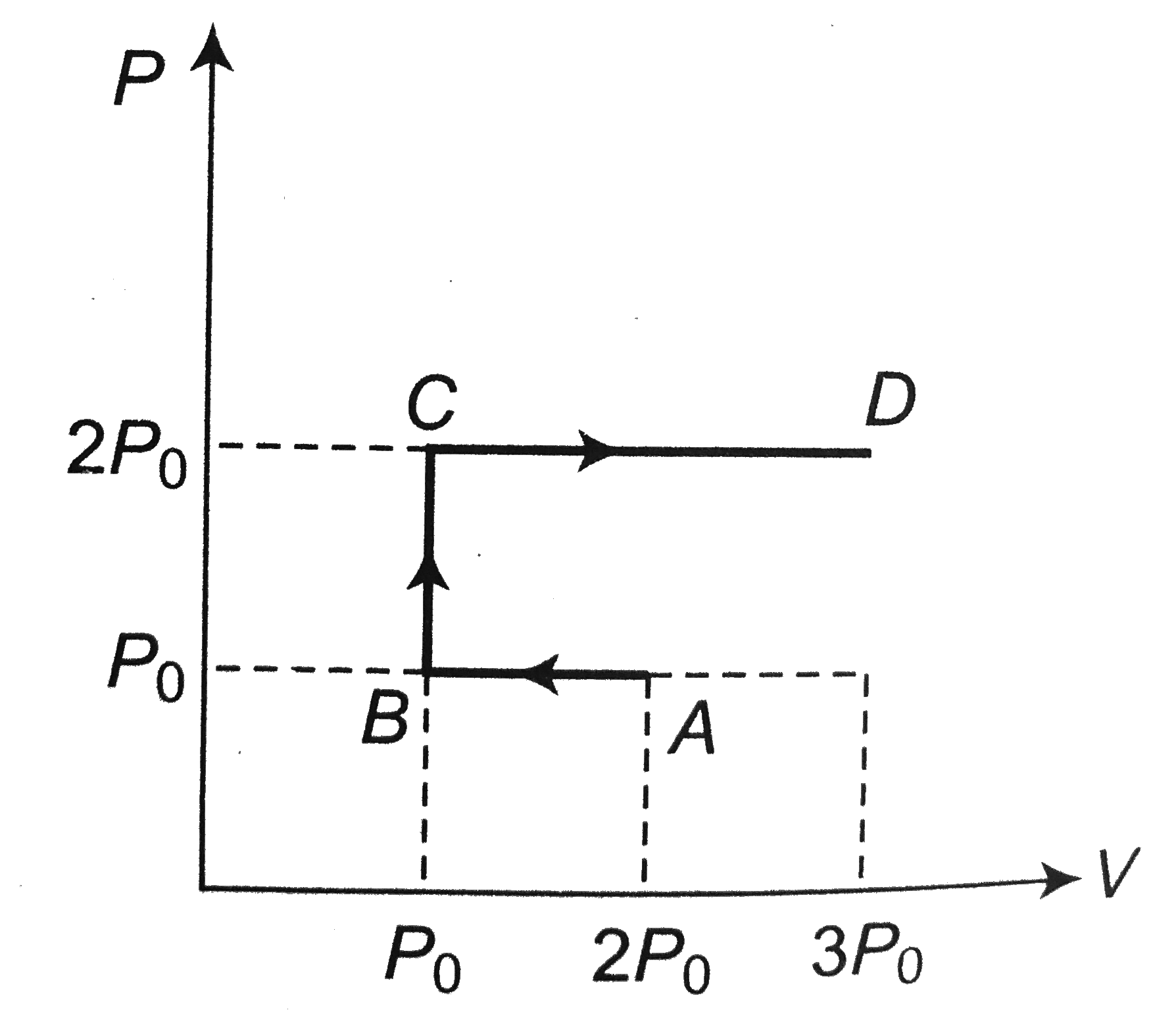
4P0V0
2P0V0
3P0V0
P0V0
The correct Answer is:C
Step by step video, text & image solution for P - V diagram of an ideal gas is as shown in figure. Work done by the gas in process ABCD is by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMODYNAMICSBook:CENGAGE PHYSICSChapter:THERMODYNAMICSExercise:8
Explore 3 Videos
- THERMODYNAMICSBook:CENGAGE PHYSICSChapter:THERMODYNAMICSExercise:9
Explore 3 Videos
- THERMODYNAMICSBook:CENGAGE PHYSICSChapter:THERMODYNAMICSExercise:6
Explore 3 Videos
- SUPERPOSITION AND STANDING WAVESBook:CENGAGE PHYSICSChapter:SUPERPOSITION AND STANDING WAVESExercise:Comprehension Type
Explore 5 Videos
- TRANSMISSION OF HEATBook:CENGAGE PHYSICSChapter:TRANSMISSION OF HEATExercise:Single correct
Explore 9 Videos
Similar Questions
A gas expands with temperature according to the relation V=kT2/3. What is the work done when the temperature changes by 30∘C?
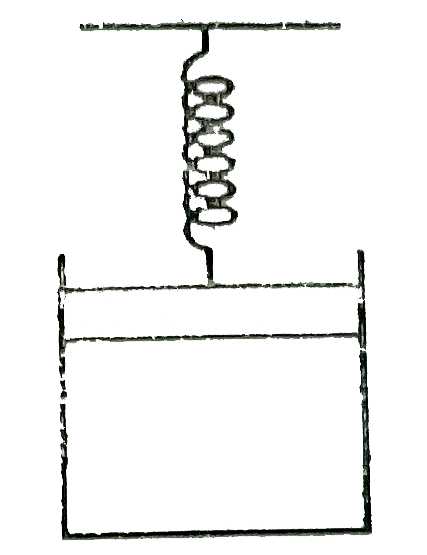
View SolutionOne mole of an ideal gas is kept enclosed under a light piston (area =10−2m2) connected by a compressed spring (spring constant 100N/m). The volume of gas is 0.83m3 and its temperature is 100K. The gas is heated so that it compresses the spring further by 0.1m. The work done by the gas in the process is N×10−1J. Find N. (Take R=8.3J/K−mole) and suppose there is no atmosphere).
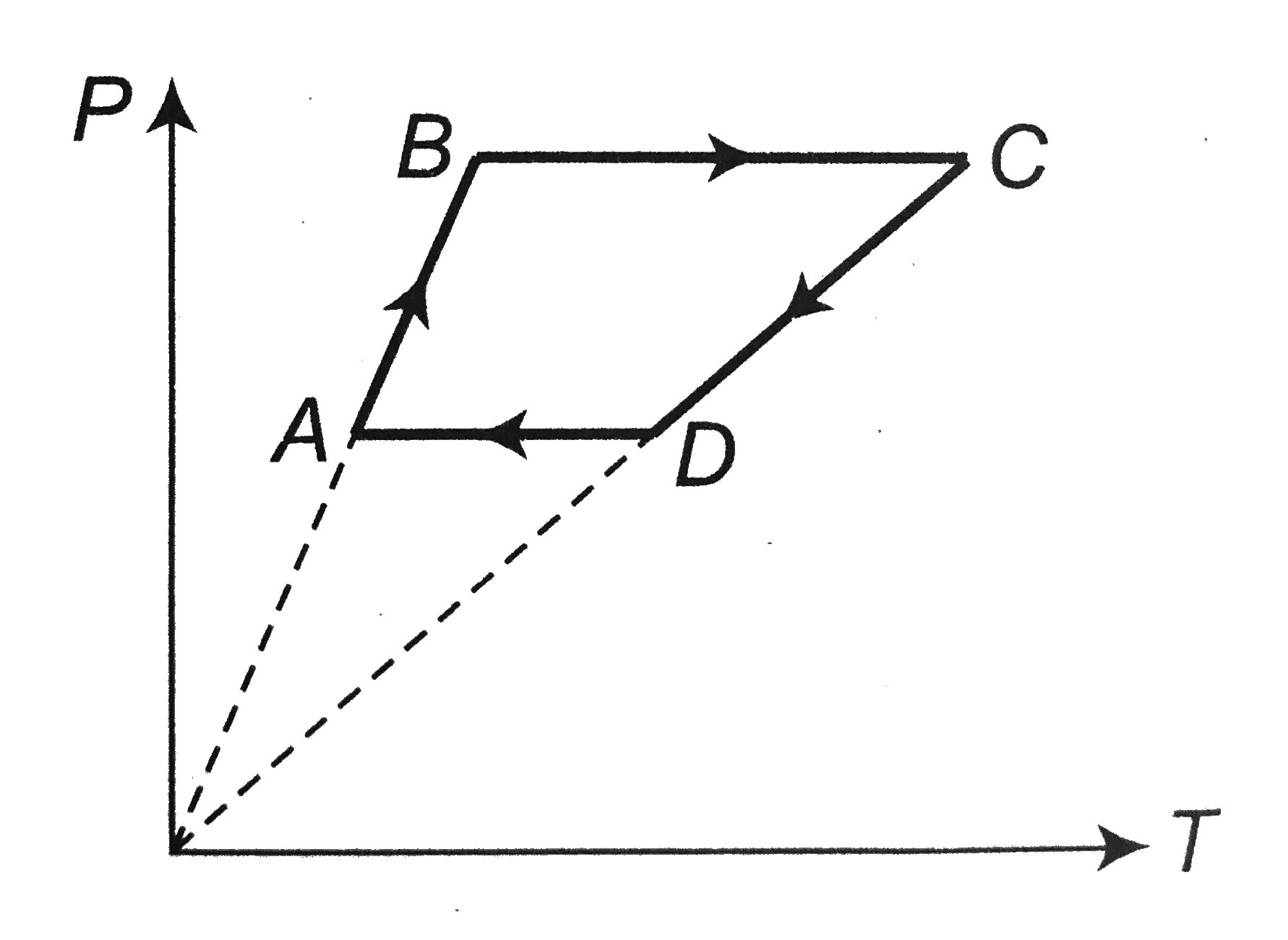
View SolutionSix moles of an ideal gas performs a cycle shown in figure. If the temperature are TD=600K, TB=800K, TC=2200K and TD=1200K, the work done per cycle is
View SolutionA string of length L, fixed at its both ends is vibrating in its 1st overtone mode. Consider two elements of the string of the same small length at positions l1=0.2Landl2=0.45L from one end.
If K1andK2 are their respective maximum kinetic energies,then
View SolutionConsider two hot bodies B1 and B2 which have temperature 100∘C and 80∘C respectively at t = 0. The temperature of surroundings is 40∘ C. The ratio of the respective rates of cooling R1 and R2 of these two bodies att=0 will be
View SolutionP−V diagram of an ideal gas is as shown in figure. Work done by the gas in process ABCD is

View SolutionAn ideal gas underoges cyclic process of ABCDA as shown in Given P−V diagram (figure)
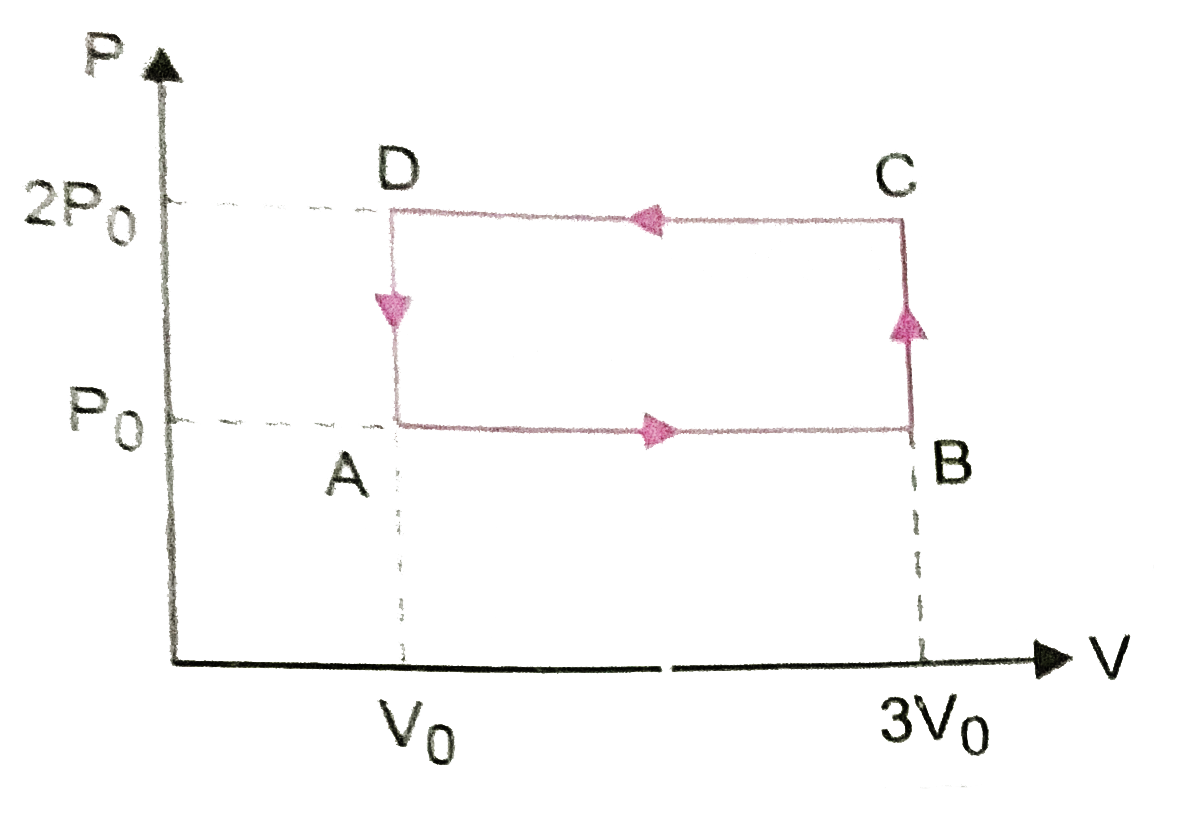
The amount of work done by the gas is
View SolutionV-T graph of a process of monoatomic ideal gas is shown in figure.
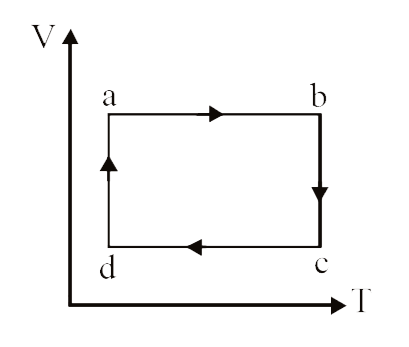
Sum of work done by the gas in process abod is-
View SolutionAn ideal gas undergoes cyclic process ABCDA as shown in givend p−V diagram. The amount of work done by the gas is
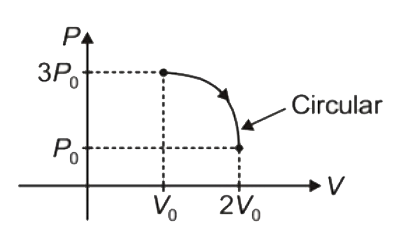
View SolutionOne mole of an ideal diatomic gas is taken through a process whose P−V diagram is shown in the figure. The work done by the gas is
View SolutionP – V graph of an ideal gas is as shown in the diagram . Work done by the gas in the process ABCD is
View SolutionP−V diagram of an ideal gas is as shown in figure. Work done by the gas in process ABCD is

View Solutionचित्र में, एक आदर्श गैस के P-V वक्र को दर्शाया गया है। गैस द्वारा प्रक्रम ABCD में किया गया कार्य है
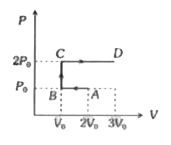
View SolutionAn ideal gas undergoes cyclic process ABCDA as shown in given P - V diagram. The amount of work done by the gas is ___
View SolutionP-V diagram of an ideal gas is as shown in figure. Work done by the gas in the process ABCD is
View SolutionAn ideal gas undergoes cyclic process ABCDA as shown in givend p-V diagram. The amount of work done by the gas is
View Solution
